Melanoma is widely known as the most lethal skin cancer. Specific tumor-related mortality can be significantly reduced if diagnosis and treatment are properly performed during initial phases of the disease. The current search for bio-markers in early-stage melanomas is a high-priority challenge for physicians and researchers. We aimed to assess the immunoexpression of BRAFV600E and KIT in a case series consisting of 44 early-stage melanomas. Formalin-fixed paraffin-embedded samples were systematically evaluated using a semi-quantitative method based on scores of percentage and intensity for immunostained tumor cells. We observed significant concordance between BRAFV600E and KIT immunoexpression in thin invasive melanomas. Our findings corroborate previous evidence showing abnormal expression of proteins associated with MAPK intracellular signaling pathway in early-stage melanomas.
Cutaneous melanoma (CM) is the world’s most lethal type of skin cancer.1 In Brazil, estimates indicate 6,260 new cases in 2018, most of which in the southernmost states.2 Mortality can be significantly reduced if diagnosis and treatment are properly performed during initial phases of the disease.3
The tumor may arise de novo or from preexisting or atypical nevi.4 It is usually related to intermittent sun exposure and/or family history.4 Early-stage melanomas can be divided into melanoma in situ (MIS) and thin invasive melanoma (TM). MIS is the first stage of histologically detectable malignant melanocytic lesion and is characterized by lentiginous proliferation of atypical melanocytes.5 TM is defined as invasive disease with thickness less than 1.0 millimeter.6
Biologically, CM is a complex disease influenced by genetic and environmental factors. Stepwise acquisition of mutations leads to activation of oncogenes and inactivation of tumor suppressor genes.4 Constitutive activation of the mitogen-activated protein kinase (MAPK) pathway represents a crucial pathogenic mechanism in several types of cancer. 7 Murine sarcoma viral oncogene homolog B (BRAF) gene plays a major role in the regulation of MAPK pathway. Activating BRAF mutations occur in 40-50% of CM.8BRAFV600E is the most common mutation and represents about 90% of BRAF mutations.8 KIT proto-oncogene (KIT) encodes a homonymous tyrosine--kinase known to activate several signaling pathways, including the MAPK and phosphoinositide-3 kinase (PI3K) pathways. It has been suggested that KIT plays a role in the early pathogenesis of CM.9
We assessed the immunoexpression of BRAFV600E and KIT in a case series of early-stage skin melanomas and discussed our results in light of the current literature.
Material and MethodsThis retrospective study was based on formalin-fixed paraffin-embedded samples obtained from the archives of a private der-matopathology laboratory in São Paulo, Brazil. Inclusion criteria: (1) early-stage melanoma diagnosis (MIS or TM); (2) paraffin block available; and (3) sufficient amount of tumor tissue.
Immunohistochemical assays were performed in 3-micro-meter-thick whole tissue sections. Immunolabelling was scored based on percentage: absent (0), 1–10% (1), 11–50% (2), and greater than 50% (3); and intensity: absent (0), weak (1), moderate (2) and strong (3). Final score was obtained from the sum of both parameters. A positive score was defined as greater than or equal to 3. All slides were reviewed and interpreted by pathologists.
Cohen’s kappa agreement test was used to study concordance between BRAFV600E and KIT immunoexpression. The study was approved by the Institutional Review Board of Universidade Federal de São Paulo under case review CAAE 30466114.9.0000.55050.
ResultsTable 1 summarizes the clinicopathological features. Among 44 patients with early-stage CM, there were 25 women and 19 men. Mean age at diagnosis was 54 years, ranging from 23 to 92 years. Information on age was missing for one patient (case no. 6). Nineteen patients (43.1%) presented tumors located on the trunk, 12 (27.2%) on the upper extremities, and seven (15.9%) on the lower extremities. Tumor location was not available for six cases (numbers 6, 8, 9, 30, 32, and 42).
Clinical and pathological results
| TM subgroup | MIS subgroup | Total | |
|---|---|---|---|
| Number of cases (%) | 35 (79.5%) | 9 (20.5%) | 44 (100%) |
| Mean age (range) | 66,1 years (46–90) | 51,1 years (23–92) | 54 years (23–92) |
| Sex ratio | 1.5F: 1M | 1F: 1.25M | 1.3F: 1M |
| Anatomic location (%) | Trunk (45%) | Trunk and upper limbs (66%) | Trunk (43%) |
| Mean Breslow thickness (range) | 0.48 mm (0.20–0.83) | Not applicable | 30/35 cases (85.7%) < 0.76 mm |
Our early-melanoma series consisted of 35 cases of TM (79.5%) and 9 cases of MIS (20.5%). In the invasive tumors, mean Breslow thickness was 0.48 millimeters, ranging from 0.20 to 0.83 millimeters. Thirty out of 35 TM (85.7%) showed Breslow thickness less than 0.76 millimeters.
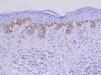
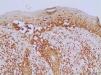
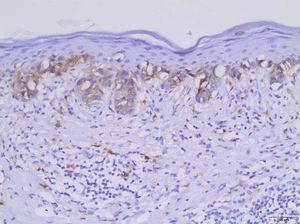
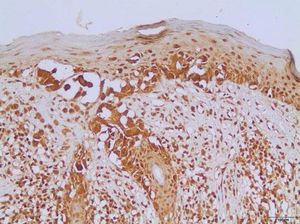
Table 2 shows the detailed immunohistochemical data. Thirty-one out of 44 cases (70.5%) expressed KIT protein. Twenty out of 40 cases (50.0%) showed anomalous expression of BRAFV600E mutated protein. Figures 1and2show representative cases. We found significant concordance (κ=0.429) between KIT and BRAFV600E immunoexpression in the TM subgroup.
In recent decades, public awareness of sun damage and its harmful cumulative effects on the human skin has contributed to a significant increase in earlier detection of CM. Early-stage disease is potentially curable with local surgical resection, while thick tumors are much more likely to behave aggressively.3 Efficient markers with major prognostic significance remain to be defined.
Several intracellular signaling pathways have been implicated in melanoma genesis and progression.4 MAPK and PI3K pathways are among the most critical ones.4BRAF and KIT mutations are regarded as primary oncogenic events in tumorigenesis, occurring in different subtypes of melanoma, cutaneous or otherwise.4
Based on the recently published molecular classification, BRAF-related tumors represent the largest genomic subgroup.8 Among the hot-spot mutations for this subgroup, BRAFV600E is the most frequent.3 Its detection has become clinically relevant after the development of BRAF and MEK inhibitors.10 BRAFV600E-mutant early-stage melanomas are strongly associated with poorer specific survival when compared to BRAFV600E-wild type tumors.11 However, its detection does not mean malignancy, since it can also be found in melanocytic nevi.12
Besides playing a major role in melanocyte survival, growth, differentiation, and migration, it has been suggested that KIT mutations are related to tumor progression of early disease.13 According to Posch et al., abnormal KIT signaling in melanoma induces to horizontal spread from the main lesion into the surrounding healthy skin.13 Authors further advocate that pharmacological inhibition of KIT-mutant cells might represent a potential strategy to reduce migratory activity, local recurrence, and disease spread.13
In this study, we found concomitant immunoexpression of BRAFV600E and KIT proteins mainly in thin invasive melanomas in a case series consisting exclusively of early-stage tumors. As previously reported by Bastian, BRAFV600E and KIT mutations are considered primary oncogenic events in the initiation of melanocytic neoplasia.4 Our findings also agree with those of Montagnani et al., who demonstrated that several point mutations such as BRAFV600E and KIT occur early during melanoma development, whereas somatic copy number alterations tend to emerge over the course of tumor progression.14 The molecular mechanisms involved in such associations remain to be elucidated. According to Neiswender et al., a key possibility is that KIT can activate signaling through wild-type RAF proteins, thus interfering with BRAFV600E-driven melanoma formation.15 Thereby, KIT inactivating mutations acquired along tumor progression would facilitate the fast-track proliferative pathway promoted by BRAFV600E.15
The present study is not free of limitations. We consider the lack of molecular genomic assessment, the small sample size, and the retrospective approach without follow-up data as the most relevant ones. The high cost of molecular methods and insufficient amount of available tumor tissue were major hurdles for us. Nevertheless, our results obtained from a previously unpublished case series consisting exclusively of early-stage tumors help further characterize and elucidate melanoma emergence and progression in the Brazilian population.
Briefly, we described the frequency of BRAFV600E and KIT immunoexpression in an early-stage melanoma series and observed significant concordance between BRAFV600E and KIT labeling in the TM subgroup. Our findings corroborate previous evidence showing abnormal expression of proteins associated with MAPK intracellular signaling pathway in early tumors.
AcknowledgmentsThe authors wish to thank Dr. Andréa Cristina de Moraes Malinverni for her support in revising the manuscript.
Financial support: This study was supported by a grant from the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP No. 2011-20435-6).
Conflict of interest: None.