Chronic leg ulcers affect a large portion of the adult population and cause a significant social and economic impact, related to outpatient and hospital care, absence from work, social security expenses, and reduced quality of life. The correct diagnosis and therapeutic approach are essential for a favorable evolution.
ObjectiveTo gather the experience of Brazilian dermatologists, reviewing the specialized literature to prepare recommendations for the diagnosis and treatment of the main types of chronic leg ulcers.
MethodsSeven specialists from six university centers with experience in chronic leg ulcers were appointed by the Brazilian Society of Dermatology to reach a consensus on the diagnosis and therapeutic management of these ulcers. Based on the adapted DELPHI methodology, relevant elements were considered in the diagnosis and treatment of chronic leg ulcers of the most common causes; then, the recent literature was analyzed using the best scientific evidence.
ResultsThe following themes were defined as relevant for this consensus - the most prevalent differential etiological diagnoses of chronic leg ulcers (venous, arterial, neuropathic, and hypertensive ulcers), as well as the management of each one. It also included the topic of general principles for local management, common to chronic ulcers, regardless of the etiology.
ConclusionThis consensus addressed the main etiologies of chronic leg ulcers and their management based on scientific evidence to assist dermatologists and other health professionals and benefit the greatest number of patients with this condition.
The prevalence and incidence of chronic ulcers is increasing with the aging of the population and higher prevalence of associated chronic conditions, such as systemic arterial hypertension and diabetes mellitus.1 Many diseases manifest themselves as chronic ulcers, especially those of the legs, which occur below the knee, do not heal within six weeks, and cause a significant social and economic impact.2
The most common etiologies are venous, arterial, and neuropathic, corresponding to 90% of the causes; however, the hypertensive etiology is also relatively frequently. These ulcers will be discussed in this consensus, focusing on the diagnosis and specific management of each etiology, and the general principles for their approach, with the recommendations of Brazilian specialists in dermatology.
MethodsSeven specialists from six university centers with experience in chronic leg ulcers were appointed by the Brazilian Society of Dermatology to reach a consensus on the diagnosis and therapeutic management of these ulcers using the adapted DELPHI methodology. In the first phase, the topics that would be addressed were defined; subsequently, the themes were divided by expertise for a search in the recent literature, with emphasis on treatment recommendations available in Brazil. Consensus was defined as approval by at least 70% of the panel members.
Results and discussionThe following themes were defined as relevant for this consensus: the most prevalent differential etiological diagnoses of chronic leg ulcers, including venous, arterial, neuropathic, and hypertensive ulcers. It was also decided to address the topic of general principles for the local management of injuries, as they are common to all chronic ulcers, regardless of their etiology.
Table 1 lists the causes of chronic skin ulcers, with emphasis on those that occur on the legs.
Main causes of chronic skin ulcers.
| Infectious | Bacterial (bullous erysipelas, necrotizing fasciitis, botryomycosis, gas gangrene, ecthyma gangrenosum, septic embolism, bacterial endocarditis, carbuncle [Bacillus anthracis], diphtheria, meningococcemia, bartonellosis, glanders, tularemia, and yaws) |
| Mycobacteriosis (leprosy, Buruli ulcer, tuberculosis) | |
| Viral (herpes simplex, varicella zoster, cytomegalovirus) | |
| Fungal (bullous tinea pedis, eumycotic mycetoma, chromomycosis, coccidioidomycosis, sporotrichosis, histoplasmosis and paracoccidioidomycosis) | |
| Protozoa (leishmaniasis, amoebiasis) | |
| Drug-induced | Hydroxyurea, methotrexate, chemotherapy, warfarin |
| Neoplastic | Metastasis of internal malignancies, squamous cell carcinoma (Marjolin's ulcer) |
| Basal cell carcinoma; | |
| Melanoma, Merkel carcinoma, Kaposi's sarcoma | |
| Malignant fibrous histiocytoma, lymphoproliferative diseases | |
| Systemic diseases | aDiabetes mellitus |
| Neuropathic (tabes dorsalis, paraplegia, multiple sclerosis) | |
| Genetic (Klinefelter syndrome) | |
| aArterial hypertension (Martorell's ulcer) | |
| Hematological (polycythemia vera, sickle cell anemia, thrombocytopenia, paraproteinemia) | |
| Autoimmune (scleroderma, rheumatoid arthritis, lupus erythematosus) | |
| Inflammatory (inflammatory bowel disease, including metastatic Crohn's disease) | |
| Nutritional deficiencies (caloric, protein, vitamins, and minerals) | |
| Primary skin diseases | Necrobiosis lipoidica, sarcoidosis |
| Pyoderma gangrenosum | |
| Panniculitis (including erythema induratum) | |
| Bullous (pemphigus, bullous pemphigoid, bullous lichen planus, porphyria cutanea tarda) Stevens-Johnson syndrome, and toxic epidermal necrolysis | |
| Related to drug abuse | Injection of illicit drugs, toxic and irritating effects of illicit or adulterated drugs, cocaine-induced vasoconstriction, bacterial embolism |
| Trauma | Burns, bites, post-surgical |
| Factitious | Dermatitis artefacta, disease simulation, Munchausen syndrome |
| Vascular | aVenous ulcers: chronic venous insufficiency, congenital valve insufficiency, post-traumatic valve insufficiency, mixed venous-arterial or venous-lymphatic insufficiency, arteriovenous malformation |
| a Arterial ulcers and thromboangiitis obliterans | |
| Vasculitis | Small vessel vasculitis: leukocytoclastic, microscopic polyangiitis, granulomatosis with polyangiitis (Wegener's granulomatosis), Churg-Strauss, Henoch-Schönlein purpura, cryoagglutination (cryoglobulinemia, cryofibrinogen), and Behçet's disease |
| Medium-size-vessel vasculitis: polyarteritis nodosa, anti-phospholipid antibody syndrome | |
| Vasculopathy | Hypercoagulability disorders |
| Disseminated intravascular coagulation and purpura fulminans, Sneddon's syndrome (usually presents with livedo reticularis), cholesterol emboli | |
| Calciphylaxis | |
| Warfarin-induced necrosis (and heparin-induced necrosis), livedoid vasculopathy, Degos disease (malignant atrophic papulosis) |
Adapted and translated from Morton & Phillips.13
Venous ulcers (VU) occur in the most advanced stage of chronic venous disease.
Clinical features that aid in diagnosisIts main clinical features3,4:
- •
Shape: Irregular, superficial in the beginning, and deepening as it evolves, with well-defined edges and commonly with yellowish exudate. The ulcer bed may have devitalized and colonized tissue; necrosis is rarely observed.
- •
Location: Distal portion of the legs (gaiter area), particularly on the medial malleolus region and rarely occurring on the upper calf and feet.
- •
Skin around the ulcer: Purpuric and hyperpigmented (ochre dermatitis); eczema can occur, evidenced by erythema, vesicles, flaking, pruritus, and exudate; varying degrees of induration and fibrosis indicate lipodermatosclerosis or fibrosing panniculitis, which can occur with or without ulcers; atrophic stellar scars of an ivory white color can be observed, with surrounding telangiectasias (atrophie blanche), located mainly on the distal third of the leg.
- •
Varicose veins and leg edema may be present.
- •
Pain: When present, it is of variable intensity; in general, it worsens at the end of the day with the orthostatic position and improves with limb elevation.
- •
Peripheral pulses: It is important to palpate the posterior tibial pulse and dorsalis pedis pulse; pulses should be present, but when decreased or absent, an association with arterial disease should be investigated.
Objective tests may be necessary to confirm the diagnosis, determine the etiology, locate the anatomical site of the venous disease (superficial, deep, and perforating venous system) and the severity of the disease, or identify coexisting peripheral arterial disease.5
The main recommended complementary exams are:
- a)
Ankle-brachial index (ABI): it is important when there are doubts about the coexistence with arterial disease, i.e., reduced or absent peripheral leg pulses. It is the reason for the higher value of systolic blood pressure in the ankle when compared with the systolic blood pressure in the brachial artery. An ABI < 0.9 indicates arterial insufficiency component, influencing the onset of the ulcer. In elderly patients and/or with diabetes mellitus, when ABI > 1.2, the hallux/brachial index should be calculated; values > 0.6 suggest adequate arterial flow.
- b)
Duplex venous mapping: the non-invasive exam of choice to assess the superficial, deep, and perforating venous system; it allows functional assessment, i.e., to identify whether the venous disease is due to reflux, obstruction, or both.6
Phlebography, venous angiotomography, and venous angioresonance are indicated in specific cases, especially when duplex venous mapping is not conclusive.
TreatmentThe treatment of VU involves measures to eliminate or reduce the effects of venous hypertension (compression therapy, surgical treatment for venous abnormality), local treatment of the ulcer, systemic drugs that aid healing, and complementary measures.
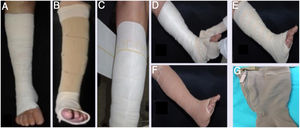
Compressive therapiesThey are the first treatment line for VU.7 They apply external pressure on the limb, which in turn improves venous hemodynamics. The external pressure that the compression must apply to the ankle is around 35 to 40 mmHg, and is gradually lower in the region below the knee. To achieve the benefits of compression, the patient must be encouraged to walk. The most widely used methods available are compressive bandages (Fig. 1A-F) and high-compression elastic stockings (Fig. 1G). High-compression stockings can be used if the ulcers are not very large. After the ulcers heal, compression stockings with a pressure of 30 to 35 mmHg are essential to prevent recurrence (Table 2).4
Main characteristics of the compression methods used to treat venous ulcers.
| Inelastic compression bandages |
| Provides high pressure during walking and small pressure at rest. |
| May remain in place for up to seven days, but presents changes in pressure over time. |
| Requires trained health staff for its placement. |
| Examples: Unna boot - Viscopaste®, Flexi-dress® (Fig. 1A); a variant is the Velcro system (Circaid® (Fig. 1B) that can be applied daily by a caregiver after adequate training. |
| Single layer elastic compression bandages |
| Greater stretch than inelastic strains and provides high pressure both while walking and resting (Fig. 1C). |
| May be applied by the patient or caregiver. |
| Examples: Surepress®, Tensopress® |
| Multilayer compression elastic bandages (two to four layers) |
| Provides sustained high pressure over time and can remain in place for up to seven days. |
| Requires a trained health staff for its placement. |
| Examples: Coban-2®, Dyna-flex® (first layer, for absorption, is composed of viscose fibers - Fig. 1D; second layer is the elastic compression bandage – Fig. 1E; third layer is an adhesive and cohesive compression bandage – Fig. 1F). |
| High compression elastic stockings |
| Ulcer kits: consists of two compression stockings, the first offers mild compression (15 to 20 mmHg) and is used to keep the dressing in place and remain overnight, while the second provides greater compression (20 to 32 mmHg) and must be placed on top of the previous one during the day (examples: Ulcer X Sigvaris®). |
| Zippered socks (Fig. 1G): to facilitate application, offering ankle pressure of 30 − 40 mmHg or 40 − 50 mmHg (examples: Ulcercomfort Venosan®, Ulcercare®). |
Important information related to compression therapy:
- a)
According to a systematic review, the multilayer system is more effective than single layer systems.8
- b)
The choice of the method depends on several factors: availability of the resource, adaptation of the patient/caregiver, cost, and adverse events.
- c)
All compression methods are contraindicated if the patient has severe peripheral arterial disease.
- d)
The use of compressive therapy is limited by pain, excessive exudation, and difficulty in application.
Although not in the scope of this consensus, it is important to comment that surgical correction of the underlying venous disease should be performed whenever possible, as surgery can promote healing, in addition to improving long-term prognosis due to the lower rate of VU recurrence.9
Local ulcer treatmentIn addition to compressive therapy, local treatment includes cleansing, debridement techniques, and dressings that minimize infection/colonization and facilitate healing. These approaches are described in the topic “General principles for local management of chronic ulcers.”
It is important to note that in cases that do not respond to standard clinical treatment, skin autograft is an alternative. Although this therapy promotes healing in many cases, it is controversial in the literature as an exclusive measure, since the frequency of ulcer recurrence is high.10
Systemic drugs that aid healingSome systemic drugs can increase the rate of VU healing. The following are recommended as treatment adjuvants:
- a)
Drugs that affect the venous tone or phlebotonics: natural and synthetic flavonoids (diosmin – 1 g/day). A systematic review concluded that these drugs improve symptoms and edema related to chronic venous disease; however, no improvement in healing was observed.11
- b)
Drugs that affect blood flow properties (hemorheological agents): the best scientific evidence is in relation to pentoxifylline, for which a systematic review showed an effective adjuvant effect with compression therapy for the treatment of venous ulcers at a dose of 800 mg three times a day.12
- a)
Rest: Decreases the effects of venous hypertension. It should be performed with the leg elevated above the level of the heart, around three to four times a day, for at least 30 minutes.
- b)
Walking: Short walks, three to four times a day, should be stimulated, as they improve the action of the calf muscle pump.
Arterial disease is responsible for approximately 25% of leg ulcers.13 These lesions arise as a result of an inadequate arterial blood supply. Its most common cause is atherosclerotic disease, however thromboembolism can cause cutaneous infarction and lead to ulceration.14
Smoking, diabetes mellitus, advanced age, and history of arterial disease (both family and personal history in other locations) are considered risk factors.14
Clinical features that aid in diagnosisSymptoms of intermittent claudication, although typical of arterial disease, may go unnoticed due to a relative tendency to immobility in these patients. They are usually painful ulcers, even when small in diameter, with worsening pain when elevating the limb and some relief when placing it in a hanging position.14
These ulcers are usually located on the lateral or pre-tibial portions of the legs, as well as on the back of the feet or on bony prominences. Classically, they have a rounded shape, a well-demarcated border, a pale and sometimes necrotic bottom, and minimal or absent exudate.13–15 The extremities are cold, the capillary filling time is slow (> 3 − 4 seconds), and peripheral arterial pulses are very reduced or absent.13,16
As a consequence of arterial hypoperfusion, trophic changes can be observed, such as pale, thin, scaly skin, with thinned hair and thickened nails.14,15
Complementary diagnosisWhen resources are limited, the diagnosis of arterial disease by manual palpation of the pulses can be considered a reliable method.17 However, in diabetic patients, the presence of a palpable pulse does not completely rule out peripheral arterial disease.
ABI measurements are also considered valid as criteria for the severity of peripheral arterial disease. ABI < 0.9 indicates peripheral arterial disease, and values < 0.5 are associated with more advanced arterial involvement and with low probability of healing. If the ABI exceeds 1.2, this may reflect arterial calcification, which makes the arteries non-compressible; in these cases, the hallux/brachial index can be used.18
The measurement of transcutaneous oxygen tension (TcPO2) is a non-invasive method considered a good indicator of critical limb ischemia.17 This technique uses sensors placed on the area of interest; calluses, edema, and bony prominences should be avoided. The sensor heats the skin, causing hyperemia and facilitating the diffusion of oxygen. The measurement of PO2 in the dermis is obtained in mmHg; on the feet, values > 50 are considered normal. A value < 40 has been associated with hypoxia capable of compromising healing and values < 30, with critical ischemia. Currently, this method has even been suggested as a way to guide the choice of amputation levels.19
Arterial eco-Doppler is a little invasive and low-cost method used to confirm the diagnosis of arterial disease. Computerized angiography and magnetic resonance angiography are used in advanced peripheral arterial disease; they are important in the identification of the exact anatomical location of arterial occlusion and in the definition of revascularization techniques by the vascular surgeon.
Specific managementThe reduction of risk factors is recommended for all patients with arterial disease; it includes smoking cessation, reduction of serum lipids, and control of hypertension and diabetes, associated with antiplatelet therapy.20
Cilostazol is a vasodilator indicated for the treatment of intermittent claudication associated with early stages of peripheral vascular disease.21 The recommended dose is 100 mg orally twice daily; a reduction to 50 mg twice daily should be considered when there is concomitant administration of CYP3A4 inhibitors, such as diltiazem, erythromycin, ketoconazole, and itraconazole, as well as during coadministration of CYP2C19 inhibitors, such as omeprazole. It is generally well tolerated; however, common adverse effects include headache, diarrhea, and palpitations. Contraindications for use are class III or IV heart failure and a history of ischemic cardiomyopathy. It should be used with caution in patients with atrial fibrillation or flutter. It can also induce leukopenia, thrombocytopenia, and even agranulocytosis, reversible with discontinuation of the medication.21
The specific treatment of arterial ulcers is aimed at correcting the flow of arterial blood supply, which can be done through a surgical or pharmaceutical approach. In a review of the Cochrane database, updated in 2020, no evidence that topical agents or dressings can influence the healing of arterial ulcers was retrieved.22
In general, surgical debridement of arterial ulcers should be avoided, as it causes a greater demand for oxygen in the adjacent tissue, inducing hypoxia and potentially contributing to increased necrosis and wound size. Irreversible tissue loss (dry gangrene or eschar) must be left dry, as moisture can make the wound the ideal medium for bacterial growth. The vascular surgeon may opt for debridement at the time of revascularization, under appropriate antibiotic coverage.20
In the case of advanced stage arterial ulcers, the main therapeutic focus is on reducing pain and preserving the leg. The first line of treatment is revascularization, both by endovascular procedures and by open surgery. However, in one-third of the patients, revascularization procedures are not possible, have little chance of success, or are not effective. In such cases, prostaglandin E1 derivatives may be indicated.23
Hyperbaric oxygen therapy is an adjuvant treatment in patients who cannot undergo reconstruction or who did not present healing despite revascularization. A Cochrane review concluded that hyperbaric oxygen therapy significantly reduced the risk of amputations in diabetic patients, provided it was performed as part of an interdisciplinary wound care program.24
Indications for emergency interventionAttention to clinical and laboratory signs of infection should be increased in cases of patients with arterial disease. Signs such as increased ulcer size, temperature, exudate, erythema, odor, and edema, in addition to the appearance of new ulcers, indicate infection.20 If at least three of these signs are present, systemic antibiotic therapy should be indicated.
Peripheral arterial disease increases the risk of infection by multi-resistant bacteria and amputation, especially in elderly and diabetic patients.25 An increase in pain, as well as clinical worsening and signs of ischemia, may be an indication for surgical intervention (angioplasty or by-pass).20
Neuropathic ulcersThe most prevalent causes of neuropathic ulcers (NU) are diabetes, leprosy, and alcoholic neuropathies.26 However, the differential diagnoses include syphilis, myelodysplasia, sarcoidosis, HIV and HTLV infection, hereditary disorders such as familial amyloidosis, and familial ulcerative-mutilating acropathy (Thevenard syndrome), among others.27,28
Clinical features that aid in diagnosisClinical manifestations of NU begin even before the ulcer itself is established. Disorders of autonomic nerves cause skin changes such as dry and thick skin and, as a consequence, fissures are observed in the plantar regions.1 The decrease in tactile, pain, and proprioceptive sensitivities, alterations in gait, and even paralysis in the most severe cases with involvement of motor nerves, lead to the formation of calluses, especially in the points of greatest support, which, if not treated correctly, can culminate in ulcers.29,30
It is important to observe whether there are deformities in the feet, such as claw toes, changes in the arching, and signs of Charcot arthropathy.30 The physician should inquire about the time of onset of the manifestations, in addition to ruling out congenital and constitutional deformities.
It is essential to assess limb perfusion, as NU can be associated with ischemic ulcers, especially in diabetic patients. In exclusive neuropathic ulcers, the pulses are generally preserved and wide. The color of the feet is normal or even erythematous (due to an autonomic disorder), being distinct from the pale and cyanotic feet observed in patients with ischemia.29,31
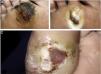
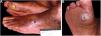
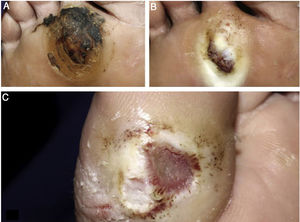
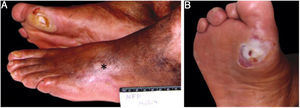
The ulcer is always preceded by non-painful calluses, which can develop purplish and/or blackened spots indicative of tissue distress and necrosis. The ulcer presents with a hyperkeratotic ring around it, forming a callous border; the center is deep and grainy. Hemorrhagic areas, indicating trauma or local friction, are observed.32 The most frequent location is the plantar region, mostly at the support points, equivalent to the foot triangle, generally on the forefoot at the first and fifth metatarsals and on the calcaneus, in addition to the plantar regions of the distal phalanges of the toes (Figs. 2 and 3).
The main complications of NU are local skin infections and osteomyelitis.32,33 The signs of infected NU are a foul odor, presence of yellow-greenish exudate, the appearance of local pain, and the presence of excess slough. Ulcers with necrosis are more prone to infection. In turn, osteomyelitis can be silent and manifest as an ulcer that is difficult to heal and exudate output, in addition to the presence of pain due to activation of deep periosteal nerve endings.33
There may be varying degrees of sensory, autonomic, and motor changes. The main complaints of patients are burning, tingling, sharp pain, limb edema, and loss of sensation. In more advanced cases, difficulty in walking, loss of shoes while walking, or even musculoskeletal deformities due to sequelae have been reported.27,32,33
Complementary diagnosisThe main tools are anamnesis and physical examination.30 The evaluation of these ulcers depends on a thorough dermato-neurological examination, as well as on knowledge and application of the anatomy and physiology of peripheral nerves.
Symptom onset time should be characterized as acute (< 1 month), subacute (between 1 and 3 months) or chronic (> 3 months).30 The type of neuropathy also contributes to the investigation of the cause; it may be intradermal, mononeuropathy, multiple mononeuropathies, or polyneuropathy. In addition, the types of nerve fibers affected are of fundamental importance in the diagnosis: sensory (pain, temperature, vibration, tactile, and kinetic-postural), autonomic, and motor fibers.26,28,30
The main sensitivities that can be tested are: tactile, which is evaluated through Semmes-Weinstein nylon monofilaments (esthesiometer) and allows semi-quantitative assessment, essential for diagnosis and follow-up; pain, which can be tested with a needle and is more sensitive than the tactile sensitivity test;29,30 thermal, which can be assessed using test tubes filled with hot and cold water; and the vibration sensitivity, using a 128 Hz tuning fork. All of these sensitivity tests should be performed symmetrically, in homologous regions.30
Trophic changes usually present as muscle hypotrophy. The neurological assessment may indicate the presence of hyporeflexia.30 Motor changes are graded through a manual strength test from 0 to 5, where 0 represents complete paralysis without moving the joint, 1 is the minimum degree of muscle contraction, and 5 represents maximum normal muscle strength.34
The most appropriate laboratory and complementary exams to assess neuropathies are those for the diagnosis/monitoring of diabetes.33 The assessment of neuropathy secondary to leprosy begins with the search for areas with altered sensitivity in the skin that may or may not coincide with hypochromic macules or erythematous-infiltrated plaques, and palpation of peripheral nerves that may show asymmetries and/or focal changes regarding thickening, pain, and shock. Peripheral nerve ultrasound can be used to demonstrate asymmetric or focal thickening, especially in the ulnar nerves,35 whereas in diabetes mellitus, nerve palpation is painless, with symmetrical and non-focal thickening.35,36
Electroneuromiography is important for the differential diagnosis: a symmetrical and diffuse polyneuropathic pattern is observed in diabetes mellitus, while a pattern of multiple asymmetric and focal mononeuropathy is observed in leprosy. Such exams contribute to the diagnosis and monitoring of the neuropathy in question.37
Specific managementTreatment should start with prevention. This includes daily inspection of the feet, cleaning and drying of the interdigits, straight nail cutting, hydration, lubrication (avoiding the interdigits), foot massage, restriction to walking barefoot, monitoring sensitivity with monofilaments, removing calluses, and examination of the pulses and of the presence of deformities of the feet.38,39 Physicians should recommend the use of shoes without internal seams, made of comfortable fabrics and usually a size larger, in addition to seam-free socks, in order to avoid any possibility of pressure points.
In patients with neuropathy already installed, the approach must be multidisciplinary; the treatment of the underlying disease is important, as well as the evaluation of shoes and orthoses/insoles to avoid repetitive local trauma.38 Diabetes patients must maintain strict glycemic control; leprosy patients must undergo the usual and indicated treatment, in addition to treating reactions early.30 Patients with alcoholic neuropathy or other hypovitaminosis should receive adequate vitamin replacement and cease the habits that cause the deficiencies.33,38
Physiotherapeutic support is important for the early detection of risk areas, with functional assessment for early diagnosis of sensory and motor loss,40 which contributes to the promotion of the patient’s health, with muscle strengthening, balance, and proprioception practices aiming at improving and maintaining the sensitivity and musculature of the foot.40,41 The indication of insoles, orthoses, and shoes should be individualized, especially when the foot already has amputation scars, which changes its biomechanics, predisposing it to a greater risk of ulcerations and new amputations.42 Manual and electrothermal phototherapy techniques also contribute to wound healing and scarring improvement.42,43
The initial specific treatment of the NU is to remove the calluses by eliminating this “natural” pressure point, both from the center and in the peripheries of the already open ulcer.42–44 After this approach, debridement products (collagenases and fibrinases, among others) are used if there is still devitalized tissue; otherwise, dressings with calcium alginate in the center are used, covered with hydrocolloid plaque. Both are used in association with secondary dressings that offer reduced pressure points, in addition to insoles and suitable footwear. Changes of dressings vary depending on the product used, from two times a day with collagenase, to every three days with calcium alginate and hydrocolloid. However, the site should be inspected daily, due to the risk of infectious complications; in these patients, in general, the classic phlogistic signals do not appear early due to systemic and local metabolic disorders.
Although NU are generally painless, they can be associated with neuropathic pain, as in cases of chronic decompensated diabetes mellitus and in leprosy, usually after treatment, with recurrent reactional neuritis.8 For these patients, first-line treatment is amitriptyline at doses of 25 to 75 mg/day or nortriptyline at doses of 25 to 150 mg/day; the latter presents a better safety profile. Other options include duloxetine at doses of 30 to 120 mg/day and venlafaxine at doses of 150 to 225 mg/day.28
Also as a first-line treatment, gabapentin (up to 2,400 mg/day) and pregabalin (up to 600 mg/day) are considered effective in diabetic neuropathy.28
For second-line treatment, tramadol and opioids can be used. In acute neuropathic pain or flares, such medications can be used as the first line. The maximum dose of tramadol is 400 mg/day. Patients with a personal or family history of drug abuse should be counseled and are more likely to misuse such medications.28
As a third-line treatment, citalopram at doses of 40 mg/day (in the elderly, the maximum dose should be 20 mg/day) and paroxetine 60 mg/day (in the elderly, the maximum dose should be 40 mg/day) can be used.28 Carbamazepine at doses of 200 to 600 mg/day, lamotrigine 400 mg/day, and valproate up to 1,200 mg/day are other options described in the literature.28
Martorell's hypertensive ulcer (MHU)Martorell's hypertensive ulcer (MHU) is a less common and probably underdiagnosed cause of chronic ulcers of the legs.45–47
Clinical features that aid in diagnosisIt is clinically characterized by a necrotic, phagedenic ulcer with an erythematous halo. It has a rapid growth and is extremely painful, with symptoms disproportional to the size of the ulcer.19,45,47 Severe pain does not improve with elevation of the limb or rest. The most common location is the anterolateral distal region of the leg,45,50,51 followed by the Achilles tendon region (about 15% of cases).46 Ulcers can be bilateral in approximately half of the cases, and satellite lesions are common.45,46,50 By definition, patients have a long-standing and poorly controlled history of systemic arterial hypertension (SAH).49 Type 2 diabetes mellitus is the most prevalent associated comorbidity, observed in up to 60% of cases.45–47 A history of previous trauma at the site of the ulcer onset is reported by half of the patients.45
MHU has been described in patients without signs of arterial or venous insufficiency,45,49,51 and patients classically have normal ABI.45,47 However, some authors report a concomitant occurrence of peripheral arterial insufficiency in approximately 50% of patients,46,47 in addition to other conditions, such as obesity.48,52
Complementary diagnosisThe diagnosis of MHU is based on clinical characteristics, histology, and exclusion of differential diagnoses.53 Arterial and venous ulcers can be distinguished by clinical and complementary imaging tests.
Surgical biopsy should always be performed, preferably extending to the fascia.47,50 Punch biopsy can lead to an incorrect diagnosis and should be avoided.46 The most common histopathological findings are arteriosclerosis in the subcutaneous tissue, calcinosis, and occasionally hyalinosis of the middle layer of the arterioles and intimal hyperplasia.45,46,50,51 These findings are not pathognomonic and can occur in calciphylaxis. Therefore, all patients should be screened for chronic kidney disease and alterations in phosphorus and calcium metabolism.45,46,50,53
Due to its rapid growth and erythematous border, MHU is often confused with pyoderma gangrenosum. In addition to inadequate treatment with immunosuppressants, this incorrect diagnosis delays the onset of debridement and other surgical treatment modalities. Clinical diagnosis associated with histology is fundamental to distinguish between the two entities, since pyoderma gangrenosum frequently presents as an ulcer with violet undermined borders and, on histopathological examination, an abundant neutrophilic inflammatory infiltrate is observed.45–47,50,53,54
TreatmentThe treatment of MHU can be divided into systemic therapy, pain control, surgical treatment, conservative treatment, and preventive measures.45 Adequate treatment of SAH is always indicated, but it is not able to reverse the established tissue changes and act effectively in healing the ulcer.46,53 Non-selective beta-blockers are contraindicated.45 The use of oral anticoagulants such as vitamin K inhibitors and antiplatelet agents is indicated.45,53
Pain control is always challenging in the face of the excruciating pain referred by patients. Common painkillers and opioids should be used.45 However, surgical treatment of MHU is considered the first line for pain control.49
Surgical debridement, followed by partial skin grafts, promotes important pain relief and accelerates healing. It is the most effective treatment for ulcers over 3 cm in diameter.47,49 The mean post-surgical healing period is two weeks, vs. 15 months when opting for conservative treatment.49 About half of the patients need only one procedure, while others may need two or three successive grafts.50,53 It is worth mentioning that all series of cases treated with skin graft were performed in patients with MHU without associated arterial or venous disease. The literature does not feature data to allow an estimation of the success rate of treatment in patients with mixed ulcers. Negative pressure therapy can be performed after grafting in an attempt to increase the effectiveness of the procedure.45,53
In patients with chronic pain that is refractory to the therapies described above, electrical stimulation of the spinal cord and lumbar sympathectomy are described as possible therapeutic modalities, but with variable results and potentially serious side effects.46,47,51
Conservative treatment aims to remove devitalized tissues, maintain adequate humidity, and control bacterial load. It is the therapeutic modality of choice in patients with small ulcers (< 3 cm) and in ulcers that are growing.47 Compressive therapy (25 − 30 mmHg) should be instituted whenever the pain is already under control and there is no contraindication detected by ABI.45
Preventive treatment includes optimization of antihypertensive therapy, smoking cessation, skin care (such as hydration), and prevention of local trauma.45
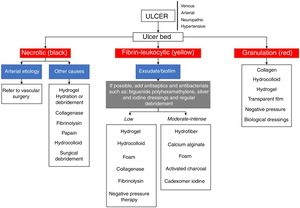
General principles for local management of chronic ulcersRegardless of the cause of the chronic ulcer, local management must be based on the knowledge of some principles, such as the TIME concept, cleaning, debridement, dressings, and biofilm control.
TIME conceptDue to the difficulty in healing a chronic ulcer, a systematic approach focused on correcting imbalances became necessary.55Table 3 describes the clinical observations and interventions related to the preparation of the ulcer bed, based on the acronym TIME (Tissue, Infection/Inflammation, Moisture, and Edge).56–58
TIME concept.
| Cause | Management | Results | |
|---|---|---|---|
| T: Tissue – Non-viable or deficient tissue | Defective ECM | Debridement (autolytic, surgical, enzymatic, biological, or mechanical) | Restoration of the base of the wound and ECM functional proteins |
| Cell debris altering healing | |||
| Negative pressure therapy | |||
| Purpose of conduct: Viable wound base | |||
| I: Infection/Inflammation | Biofilm | Biofilm control: antiseptic,a systemic antimicrobialsb | Low bacterial count |
| Prolonged inflammation | Controlled inflammation | ||
| Inflammatory cytokines | Cytokines | ||
| High protease activity | Low protease activity | ||
| Low growth factor activity | High growth factor activity | ||
| Purpose of conduct: Bacterial balance and inflammation control | |||
| M: Moisture – changes in the exudate | Lack of exudate: slows down cell migration | Hydration with hydrogel or hydrocolloid | Cell migration restored by moisture balance |
| Excess: maceration of the edges | Alginate/hydrofiber/foam | Control of excess liquid | |
| Compression therapy | Maceration prevented | ||
| Negative pressure therapy | |||
| Purpose of conduct: Moisture balance | |||
| E: Edge | No migration of keratinocytes from the edges | Reassess cause or consider auxiliary therapies: Debridement; grafts; biological agents; pharmacological therapies; other technologiesc | Keratinocyte migration |
| ECM abnormalities | Responsive cells | ||
| Abnormal protease activity | Restoration of the appropriate protease profile | ||
| Purpose of conduct: Advance the wound edge | |||
ECM, extracellular matrix.
Devitalized tissues, foreign bodies, and debris are factors that prevent the healing of wounds; its removal is essential for healing. Cleaning is the initial step in treatment and should be carried out by applying non-toxic products without damaging existing viable tissue. Chronic ulcers can be cleaned with saline solution, tap water, and polyhexanide (polyhexamethylene biguanide [PHMB]); the latter is an important tool, but its cost is high.59
- a)
0.9% saline solution: Suitable due to being isotonic, having the same pH as plasma, being hypoallergenic, and not interfering with the healing process. However, it is insufficient to clean colonized/infected tissue.
- b)
Tap water: Easy to access, but not superior to saline solution.60
- c)
PHMB: Good bactericidal efficacy; low-risk antiseptic with excellent tolerance profile, low risk of contact sensitization, and antimicrobial action in infected acute or chronic lesions. Its physical-chemical action prevents the onset of bacterial resistance.61 When associated with the surfactant betaine, it produces an autolytic debridement effect.
Povidone-iodine is a known antiseptic, but its use in the treatment or prevention of infection in ulcers is debatable, as it can cause allergy, has low penetration, and has a toxic effect on cells, disturbing tissue regeneration. However, a systematic review of randomized clinical trials62 showed that iodine did not prolong or reduce the healing time of chronic ulcers when compared with other antiseptics or dressings.
Solutions such as chlorhexidine, acetic acid, potassium permanganate, and Dakin's solution, although safe on intact skin, can be toxic to granulation tissue, by prolonging the acute inflammatory response and delaying collagen production; therefore, they are not recommended for chronic ulcers.
Debridement methodsTissue abnormalities in chronic wounds trigger the accumulation of devitalized and necrotic tissues; regular debridement is necessary to reduce necrosis and achieve healthy granulation tissue.63,64
Regular debridement helps healing by removing the biofilm, improving the biodistribution of antimicrobials, and preventing the formation of a new biofilm.65
Debridement, by definition, is any method of removing devitalized, necrotic, infected, fibrinous, or foreign material from a wound.66 The main methods are:
- a)
Surgical:
- -
Proper surgical debridement: Performed in a surgical environment under anesthesia and indicated for large areas of devitalized and/or necrotic tissue or extensive cellulitis, infected bone, or sepsis not responsive to other techniques; a rapid technique with inherent risks of bleeding and transient bacteremia, in addition to damage to structures such as nerves and tendons; requires trained medical personnel and has a higher cost.
- -
Conservative surgical debridement: Performed at the bedside, it aims to remove devitalized tissue or foreign material inside or around the wound, under local anesthesia using a scalpel, scissors, or curette. This technique is considered less aggressive and less selective when compared with the surgical technique; however, it is faster, and has similar risks of pain and bleeding in the postoperative period. Anesthesia (topical or injectable) is recommended to minimize discomfort.
- b)
Mechanical:
- Wet-to-dry: Application of gauze moistened with saline, which is left to dry and adhere to the bed of the wound, and subsequently removed by traction, removing the devitalized tissue with it. It is considered to be a low-cost, slow, non-selective, and painful method.
- Hydrotherapy: Water pressure is used in the form of a jet, directed to the surface of the lesion to remove devitalized tissue. Special care is required for ischemic ulcers.
- c)
Autolytic: Highly selective technique, performed using hydrogel and hydrocolloid dressings. It acts by retaining exudate from the wound by forming a collection of endogenous proteolytic enzymes produced by macrophages (such as collagenases, elastases, myeloperoxidases, and metalloproteinases) whose purpose is to liquefy and separate devitalized tissues from healthy tissue. This technique can cause perilesional maceration, requires minimal clinical training, is painless, and has a slow response. In order to be applied, there must be a minimum of exudate. Contraindicated in critically colonized, infected, and ischemic ulcers.
- d)
Enzymatic: It uses a topical substance containing an exogenous enzyme capable of digesting devitalized tissue. This technique requires daily changes. In Brazil, three products are available: papain, collagenase, and fibrinolysin/DNase (Table 4).67–71
Table 4.Characteristics of topical therapies with debridement action.
Types Indications Contraindications Instructions for use Papain:68 Proteolytic enzymes of papaya; in vitro antibacterial action; stimulates granulation; non-selective enzymatic debridement Any type of ulcers: with or without biofilm; with variable volume of exudate; 2% to 4% lesions in the granulation phase with variable exudate; 6% lesions with necrosis Hypersensitivity Daily exchange Maceration Local application with gauze soaked in the solution Do not place on fascia/cartilage/tendon/bone Collagenase:69 Formed by clostridiopeptidase A; it may or may not contain chloramphenicol; selectively degrades native wound collagen Safe selective enzymatic debridement on fascia/cartilage/tendon/bone; any type of ulcers Healing by first intention Daily change Allergy Apply thin layer and cover with secondary dressing Fibrinolysin:70 fibrinolysin, deoxyribonuclease, and chloramphenicol; enzymatic debridement Safe selective enzymatic debridement on fascia/cartilage/tendon/bone; any type of ulcers Healing by first intention Daily change Allergy Apply thin layer and cover with secondary dressing - e)
Biological (larvae): Uses Lucilia sericata larva; however, few studies have proved its effectiveness for VU debridement.
The most used methods in clinical practice are surgical, enzymatic, and autolytic debridement.
Biofilm controlBiofilms are structured communities of microbial cell colonies wrapped in a polymeric matrix and adhered to surfaces (natural or artificial) or to themselves. They are heterogeneous and dynamic, maintain variable genetic diversity and gene expression (phenotype), and are capable of creating environments and defenses that can produce chronic inflammation and delay healing. These colonies develop and protect themselves through the production of extracellular polymeric substances that give them structural integrity and protect them against external agents and, therefore, are characterized by resistance to antimicrobial and antiseptic agents, as well as resistance to the host’s immune system defenses. They are commonly composed of several species of microbial agents such as bacteria, fungi, and viruses.72
There is growing evidence that biofilms are present in most, if not all, chronic unhealed ulcers. In a recent meta-analysis of in vivo studies, it is highlighted that at least 78% of chronic ulcers contain them.73
It is essential to differentiate ulcers having only critical colonization or superficial infection (with biofilm), from those with a deeper infection leading to erysipelas, cellulitis, or lymphangitis.74 These are signs and symptoms that indicate infection of the tissue adjacent to the ulcer and require the introduction of systemic antibiotics: general signs such as malaise and loss of appetite, and local signs such as increased exudate, delayed healing, swelling at the base of the wound, persistent pain, friable granulation tissue, discoloration of the wound bed, foci of abscess, and fetid odor. Increased pain and wound size are probably the two most useful predictors.58
Bacteriological tests using swabs are not indicated to make this differentiation, as they qualitatively identify the presence of bacteria, but they cannot determine the quantity of bacteria, so they fail to differentiate simply colonized ulcers from those with deep infection. In cases of associated infection, when it is necessary to identify the bacteria to direct the treatment, biopsies of the ulcer base should be performed for culture after exhaustive washing of the bed with 0.9% saline.75
Suggested measures for biofilm control76:
- a)
Debridement: It is one of the most important strategies in the treatment of biofilms, but it does not remove them completely and, therefore, cannot be used alone.
- b)
Antiseptic agents, washing, or therapeutic irrigation should be used together with debridement methods to promote bacterial reduction and suppress their re-development.77 When considering topical antiseptics, those with antibiofilm properties and with less ability to induce cytotoxicity in healthy tissue are preferred. It is important to note that topical antibiotics such as neomycin, bacitracin, mupirocin, and silver sulfadiazine are not recommended for the treatment of chronic ulcers.
- c)
Topical antibiofilm therapies are described in Table 5, with emphasis on iodine alkoxide, PHMB, and silver-containing dressings.78,79
Table 5.Characteristics of topical therapies recommended for combating biofilm.
Types Indications Contraindications Instructions for use 9% cadexomer iodine 78,79: Contains 0.9% iodine; antimicrobial action breaking the lipid membrane and inhibiting bacterial protein synthesis; non-toxic to fibroblasts; the iodine is being released as the exudate is absorbed High capacity to absorb exudate; each gram absorbs 3 mL of the exudate; total/partial thickness; critically colonized/infected lesions; MSRAa Iodine allergyHashimoto thyroiditisGraves' disease Dressing exchange every 2 to 3 daysExchange when the brown color turns yellow/gray Biguanide polyhexamethylene79: Broad bacterial spectrum; its positive structure binds to the negative charges of the cell membrane, breaking its integrity; active in the biofilm; antiseptic; non toxic; non-irritating; active against MSRA,a VRE,b and fungi Reduces bacterial biofilm None Variable depending on the protocol Dressings and topical drugs containing silver79: antiseptic, anti-inflammatory and broad antibacterial action; blocks bacterial cell respiration; destroys bacterial membranes; denatures bacterial RNA and DNA; active against MSRA,a VRE,b and fungi Reduces bacterial biofilm Allergy to silverUse with caution in diabetic ulcers, due to the cytotoxic effect on fibroblasts The silver ion release method varies depending on the productThe dressing exchange is variable depending on the protocol - d)
Antibiofilm strategies should be used until the wound bed is visibly clean, presenting healthy granulation tissue and/or on the way to healing.
- e)
Systemic antibiotics are not able to eradicate biofilm from a wound; therefore, their use should be considered with caution aiming at attacking planktonic (surface) bacteria, acute infection, and prevention of associated systemic infections.
Table 6 and the flowchart of fig. 4 present information about the main dressings, such as foams, hydrogels, calcium alginate, activated charcoal/silver coverings, hydrocolloids, transparent films, Unna boots, hydrofiber, and collagen. Their indications are also described and should be used according to the characteristics of the ulcers.79–86
Characteristics of the main dressings.
| Types | Indications | Contraindications | Instructions for use |
|---|---|---|---|
| Foam79,80: Polyurethane; absorption of exudate; Maintenance of humid environment; Hydrophilic properties; Painless/atraumatic exchange | Total/partial thicknessModerate/high exudateReduces pressure and frictionProtects friable peri-ulcer skin | Third-degree burnsIschemic ulcers with eschar at the baseDry/necrotic bedUlcers with fistulas | Primary/secondary dressingaDepends on the volume of exudate (1 to 7 days) |
| Hydrogel79,81: Hydrophilic cross-linked polymers with 80%−90% water; non-adherent; autolytic debridement; absorbs minimal amounts of exudate | Total/partial thickness | Third-degree burns | Apply at the base of the ulcer and cover with secondary dressing |
| Dry lesions with minimal exudate | Moderate/high exudate | ||
| Ulcers with critical colonization and infection | Daily use | ||
| Do not use as a wound filler | |||
| Calcium alginate79,82: Derived from seaweed; exudate absorption; formation of humid environment by gel formation (calcium and sodium salts + exudate); hemostatic properties; autolytic debridement; non-stick due to gel formation | Moderate/high level of exudateTotal/partial thicknessApplied to surfaces and cavities | Dry/necrotic bedThird-degree burnsMinimal exudate | Fix with secondary dressingWhen the dressing is saturated (1 to 7 days) |
| Activated charcoal/silver82: Double layer of fibers; external: coal; internal: silver; absorption of exudate; coal: adsorption of microorganisms; silver: bactericidal action | Chronic infected and exudative ulcers | Clean, granulating, and uninfected ulcersDo not use on fascia/tendons/bones | Some charcoal dressings can not be cutFix with secondary dressingExchange depends on saturation (1 to 7 days) |
| Hydrocolloids79,83: It contains an internal self-adhesive layer and a gel-forming agent such as gelatin or carboxymethylcellulose (CMC); inner layer of hydrocolloid in foam or film; increases its thickness in contact with exudate; autolytic debridement; puts up a form barrier against pathogens | Total/partial thicknessMinimum to moderate level of exudateWound bed with granulation and necrosis | Third-degree burnsInfectionBedsores | Direct applicationAdhesive edge2.5 to 5 cm safety edgeExchange depends on exudate level (3 to 5 days)No secondary dressing required |
| Transparent films84: CMC in film form; aseptic; creation of a humid environment; exudate retention | Total/partial thicknessSecondary dressingAbrasions/graft donor areasMinimum to moderate level of exudate | High level of exudate | Minimum safety edge 2.5 cm |
| Unna's boot85: 10% zinc oxide + starch on bandage of cotton fabric; non-elastic compressive action; stabilization of hydrostatic pressure; increased resistance to infections; creation of humid environment | Venous ulcers | Ulcers of ischemic origin | Prior preparation includes Trendelenburg position for 6 to 8 hours to reduce swellingWrap the ankle/knee limb |
| Hydrofiber79,83: Fiber CMC with or without silver; absorption of very much exudate; it forms hydrophilic gelatinous substance; provides humid environment; non-adherent; facilitates autolytic debridement, granulation and epithelialization | Total/partial thicknessModerate/high exudate | Dry or low exudate volume lesions | Fix with secondary dressingExchange according to saturation (1 to 2 days) |
| Collagen79,86: Bovine, porcine or sheep source; available in gel, tapes or powder; bioabsorbable; chemotactic for the cells involved; inactivates MMP, elastase and decreases the level of inflammatory mediators; stimulates endogenous collagen; in vitro bacteriostatic properties; some versions impregnated with silver; increases the epithelialization rate | Total/partial thicknessMinimum/moderate volume of exudateUninfected lesion | Allergy to source tissue | Apply directly to the ulcer bed and cover with secondary dressingExchange depends on exudate level |
Primary dressing is the first in direct contact with the ulcer bed; secondary dressing, transparent films or gauze-like fabrics or crepe stripe; MMP, metalloproteinases.
Other promising treatments, such as electrical stimulation, negative pressure therapy, hyperbaric oxygen therapy, ultrasound, and low-level laser therapy, have been used as adjuvants in the treatment of chronic ulcers; nonetheless, their respective systematic reviews indicate that further studies are necessary to certify their effectiveness.24,87–90
Final considerationsVenous, arterial, neuropathic, and hypertensive ulcers are frequent, with an especially higher prevalence in the elderly population. The correct diagnosis of these conditions and adequate treatment, based on the best scientific evidence, are essential to reduce the negative social, economic, and quality of life impacts in affected patients.
Table 7 summarizes the main causes of chronic leg ulcers, with key points in diagnosis and treatment.
Key points in the diagnosis and treatment of the main causes of chronic cutaneous leg ulcers.
| Ulcer etiology | Diagnosis | Clinical treatment |
|---|---|---|
| Venous | Clinical: distal leg ulcer, edema, ochre dermatitis, lipodermatosclerosis, and varicose veins | Compressive therapy (high compression bands and elastic stockings) |
| Distal pulses present (posterior tibial and pedal) | Cleaning, debridement, and dressings | |
| ABI between 0.9 and 1.2 | Hemorheological and phlebotonic drugs: pentoxifylline and diosmin | |
| Duplex venous mapping: evaluates the superficial, perforating, and deep venous system | Anti-stasis measures: rest with raised limbs, walking | |
| Arterial | Clinical: very painful ulcers, especially with elevated leg, in patients with a history of claudication | Control of risk factors (smoking, reduction of serum lipids, control of SAH, DM) |
| Absent peripheral pulses | Platelet antiaggregant | |
| ABI < 0.9 (values < 0.5 indicate more advanced arterial disease) | Cilostazol | |
| TcPO2 < 40 (hypoxia capable of compromising healing) and < 30 (critical ischemia) | Cleaning and dressings (avoid surgical debridement) | |
| Arterial Doppler ultrasound | Oxygen therapy | |
| Neuropathic | Clinical: ulcer in patients with a risk factor for neuropathy (e.g.: DM, leprosy and alcoholism), preferably in the plantar region and with callous edges | Control of the underlying disease (DM, leprosy, and alcoholism) |
| Decrease plantar load: shoes and orthoses to prevent repetitive trauma | ||
| Physiotherapeutic support | ||
| Normal, decreased or absent distal pulses (the latter when associated with arterial disease) | Electrothermal phototherapy | |
| Trimming of the callous edges of ulcers and debridement agents | ||
| Hypertensive | Clinical: necrotic and painful ulcers in patients with severe and poorly controlled SAH | Control of SAH (contraindication for the use of non-selective beta-blockers) |
| Oral anticoagulants: vitamin K inhibitors and antiplatelet agents | ||
| Peripheral pulses present | ||
| Normal ABI (> 0.9) | Pain control: analgesics (opioids) | |
| Histopathological findings: arteriosclerosis and calcinosis | Surgical debridement and skin grafts | |
| Exclude calciphylaxis: screened for chronic kidney disease and changes in phosphorus and calcium metabolism | Cleaning and dressings |
ABI, ankle-brachial index; TcPO2, transcutaneous oxygen tension; SAH, systemic arterial hypertension; DM, diabetes mellitus.
This consensus addressed the diagnostic and therapeutic management of chronic leg ulcers of the most common causes, based on scientific evidence and the experience of specialists, to assist dermatologists and other health professionals, in order to benefit the greatest number of patients with this condition.
Financial supportNone declared.
Authors’ contributionsLuciana Patricia Fernandes Abbade: Elaboration and writing of the manuscript, collection, analysis, and interpretation of data.
Marco Andrey Cipriani Frade: Elaboration and writing of the manuscript, collection, analysis, and interpretation of data.
José Roberto Pereira Pegas: Elaboration and writing of the manuscript, collection, analysis, and interpretation of data.
Paula Dadalti-Granja: Elaboration and writing of the manuscript, collection, analysis, and interpretation of data.
Lucas Campos Garcia: Elaboration and writing of the manuscript, collection, analysis, and interpretation of data.
Roberto Bueno Filho: Elaboration and writing of the manuscript, collection, analysis, and interpretation of data.
Carlos Eduardo Fonseca Parenti: Elaboration and writing of the manuscript, collection, analysis, and interpretation of data.
Conflicts of interestNone declared.
How to cite this article: Abbade LPF, Frade MAC, Pegas JRP, Dadalti-Granja P, Garcia LC, Bueno Filho R, Parenti CEF. Consensus on the diagnosis and management of chronic leg ulcers - Brazilian Society of Dermatology. An Bras Dermatol. 2020;95(S1):1–18.
Study conducted at the Sociedade Brasileira de Dermatologia, Rio de Janeiro, RJ, Brazil.