Spitz nevi are usually solitary, benign proliferations of epithelioid melanocytes. Various clinical and histopathological subtypes of Spitz nevi have been reported in the literature over time. The eruptive variant of Spitz nevus has been reported in 34 cases so far. Genetic analysis was performed in 7 of them. In this report, the authors present a patient with hundreds of Spitz nevi in her body in just 2 months and review the current literature together with the genetic studies.
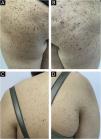
A 17-year-old young woman presented with multiple nevi starting from the buttocks and spreading to the back and legs for the last 2 months. She was otherwise healthy and had no history of any drug use. She had no complaints of itching or bleeding on the lesions. There was no freckling on her face and she did not describe photosensitivity. There was no finding of syndromes characterized by multiple lentigines, no known genetic disease in the family, and no intermittent or continuous sunlight exposure for the respective sites. In the dermatological examination, hundreds of dome-shaped nevi with reddish brown colors, <0.5 cm in diameter were observed on the legs and upper body, especially on the buttocks (Fig. 1). A few lesions compatible with Spitz nevus were observed on the neck, which were reported to have newly formed.
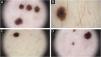
On dermatoscopic examination, there is a reticular distribution, which is darker in the center and becomes lighter in the periphery, and globules are observed in the periphery of the lesions (Fig. 2).
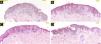
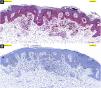
In the hematoxylin-eosin stained sections from four lesions; symmetric, junctional, localized, pigmented discohesive melanocytic nest structures with spindle cells and pagetoid spreading areas are seen (Fig. 3). No mild atypia was observed in melanocytes. Diffuse and strong positive staining was observed with S100, MelanA, HMB45. In addition, PRAME is negative, with no loss of expression with BAP-1. Thus, metastatic melanoma and Spitz nevi with loss of BAP-1 expression, which come to mind in the differential diagnosis were excluded (Fig. 4).
One lesion larger than the others was excised for genetic analysis. No mutations were detected in the HRAS gene sequence in next-generation sequencing (NextGENe and Geneticist Assistant) and the ROS1 gene in FISH analyses (CytoCell ROS1 Breakapart FISH Probe).
In the light of clinical and pathological data, the patient was diagnosed with eruptive disseminated Spitz nevus. Strong protection from sunlight and follow-up at 6-month intervals was recommended.
Spitz nevi are melanocytic lesions in dome-like morphology which are usually observed in pinkish red color, but may also be in brown tones. Among Spitz nevi, solitary forms are the most common variant. The eruptive form is extremely rare and has been reported mostly in adolescence and later. In the reported cases, the lesions generally formed and spread over months to years. Interestingly, in this case, the lesions spread very rapidly in a short period of 2 months.
Although the exact etiopathogenesis is not known, there are some theories. Some factors such as drugs, the use of cytokines, immunosuppression, inflammatory conditions, pregnancy, atopic dermatitis, and radiotherapy are considered to be responsible in the formation of lesions, it has also been reported in completely healthy people, as in this case.1–6
To date, some theories regarding the etiopathogenesis have been proposed. One view is that the nevi probably spread along the neurovascular and lymphatic networks. Sentinel lymph node biopsies have been shown as evidence for this view.7 Embryonic mosaicism has been suggested because of the cutaneous spread pattern.7 According to another view, the tropism of nevus cells to the places where lesions will occur has been suggested, and it has been stated that it waits without proliferating in the skin until a trigger factor occurs.8
HRAS, ROS1, NTRK1, ALK, BRAF and RET gene mutations have been shown in many Spitz nevus cases. The fact that these gene mutations are not encountered in all eruptive forms brings to mind that there may be other genetic pathways.7,9–11 No mutations were detected in the HRAS and ROS1 genes in this eruptive Spitz nevus case.
Table 1 presents the summary of demographic, clinical, and genetic analysis results of eruptive Spitz nevus cases that underwent genetic analysis.
General characteristics and genetic analysis results of eruptive Spitz nevus cases genetically analyzed to date (While creating this table, the article by Fernandez-Flores et al.15 was used and expanded).
| Age/Sex | Number of lesions | Localization | Formation time | Immunohystochemistry and genetic analyses | ||
|---|---|---|---|---|---|---|
| Morgan et al.12 | 30/M | Several hundred | Trunk and extremities | Last 2-years | S100 and MIB-1: low mitotic activity | Blood and nevoid fibroblasts karyotype analyse was normal |
| Boone et al.13 | 17/F | More than 50 | Head, neck, arms, thighs, and buttocks | Last 1-year | Tetraploid cells showed balanced gains in 6p25 (RREB1), 6q23 (MYB), 11q13 (cyclin D1), and Cep6, with all cells having 3 or 4 identifiable copies of each chromosomal segment | |
| Boone et al.13 | 51/F | Several | Groins, left buttock, right hip, upper thigh | Last 16-years | No gains or loses of chromosomes 11 and 16 | |
| Gantner et al.11 | 16/M | Several | Trunk, face, arms, legs and the penis | Last several months | BRAF, HRAS, KRAS, and NRAS hotspot mutations | |
| No mutations of the BRAF, RAS and CDKN2A genes | ||||||
| Feito-Rodríguez et al.14 | Several months/F | Multiple | Extremities | From the tenth day of birth | No mutation was detected in the comparative genomic hybridization analysis | |
| Raghavan et al.7 | 49/F | More than 100 | Buttocks, trunk, extremities. | For 4-years after diagnosis of Spitz nevus | TMP3-ROS1 rearrangement with identical intronic breakpoints | |
| Loss of chromosome 12q | ||||||
| Fernandez-Flores et al.15 | 29/F | Dozens | All over the body | Last 19-years | PRAME-ROS-1-PDL-1- panTRK- ALK- | Next-generation sequencing: No NTRK1, NTRK2, or NTRK3 fusions |
| FISH for PTEN showed no alteration | ||||||
| Our case | 16/F | Hundreds | Spreading from the hips to the legs and trunk | Last 2-months | HMB45+ S100+ MelanA+ PRAME−, no loss of expression with BAP-1 | No mutations were detected in the HRAS gene sequence in next generation sequencing and in the ROS1 gene in FISH analyzes. |
In conclusion, the etiopathogenesis of eruptive Spitz nevus has not been fully elucidated. Considering the age range of the affected people; the idea that Spitzoid melanocytes proliferate as a result of an as-yet-unknown trigger on the appropriate genetic background seems plausible. The fact that there is a great difference in the speed of formation of nevi in the reported reports may indicate different processes in different people rather than a single pathophysiological process. There is already a wide variety of results in genetic analysis. Much more study is needed to fully understand this phenomenon.
Financial supportNone declared.
Authors’ contributionsEmre Zekey: Literature searching, designing and writing the manuscript.
Seher Darakcı: Histopathological examination.
Conflicts of interestNone declared.
The authors would like to thank Prof. Dr. Cuyan Demirkesen for her help during the histopathological examination and photographing.
Study conducted at the Department of Dermatology and Pathology, Sivas Numune Hospital, Sivas, Turkey.