Background: In-vitro studies showed that Leucine-rich glioma inactivated 3 (LGI3) is a keratinocyte-derived cytokine that stimulates melanin synthesis and is increased after ultra violet B (UVB) irradiation. So, we postulated that LGI3 may be involved in vitiligo aetiopathogenesis and may participate in narrow band ultra violet B (NB-UVB) induced pigmentation in vitiligo.
Objectives: To assess this hypothesis, lesional LGI3 immunohistochemical expression of vitiligo patients before and after NB-UVB phototherapy was studied, and its correlation with repigmentation was evaluated.
Methods: Forty vitiligo patients and 20 age, sex, and skin phenotype-matched controls were enrolled. Patients were treated with NB-UVB thrice weekly for 12 weeks. VASI score was evaluated before and after NB-UVB sessions. For vitiligo patients, baseline LGI3 immunohistochemical staining was estimated, and compared to that of controls and to its post-treatment data in those patients. Results: Baseline LGI3 immunohistochemical studied parameters (expression, intensity, percentage and H score) were significantly lower in vitiligo cases than controls (p=0.003, 0.013, 0.001 and 0.001 respectively). After 12 weeks of NB-UVB phototherapy, these LGI3 immunohistochemical parameters were up-regulated and became comparable to that of controls (p >0.05 for all). There was a significant positive correlation between the improvement of both VASI score and LGI3 H score mean values (r=-0.349 , p=0.027).
Study limitations: Small number of investigated subjects.
Conclusion: Decreased LGI3 protein may play an active role in vitiligo pathogenesis and its up-regulation after NB-UVB phototherapy, may actively participate in NB-UVB photo-induced melanogenesis.
Vitiligo, the most common acquired variety of leukoderma, affects nearly 0.5% - 2% of the population worldwide, irrespective of ethnic origin or race, and causes significant social and psychological complications in all affected patients.1
Due to its multifactorial nature, the pathogenesis of vitiligo is poorly well-known.2 Many pathogenic theories have been postulated, including cytotoxic, autoimmune and oxidant-antioxidant mechanisms, as well as an intrinsic defect of melanocytes. Also, in segmental vitiligo, a neural hypothesis was proposed. Although all these hypotheses are attractive, it is likely that vitiligo is a result of the convergence of several ones.3
The hallmark of vitiligo is the presence of disfiguring white skin patches as a result of epidermal melanocyte damage. It was revealed that the depigmented vitiligenous areas of skin contain melanocyte progenitors and individual melanocytes, and the interaction of melanocytes with surrounding keratinocytes plays an essential role in their viability and function.4
Leucine-rich glioma inactivated 3 (LGI3) is a secreted protein having a leucine-rich repeat. It was chiefly investigated in the brain due to its high level of expression there.5 In the murine brain, LGI3 was involved in neuritogenesis, neural exocytosis and neural differentiation.6 Additionally, it is involved in amyloid peptide uptake by astrocytes in aged monkey brain.7 However, LGI3 in adipose tissues acts as adipokine that regulates adipogenesis.8
In human skin, LGI3 is expressed frequently by epidermal keratinocytes and was suggested as a novel cytokine having a keratinocyte cytoprotective effect.6 Moreover, Jeong et al., in their in vitro study, revealed that LGI3 is a potent melanogenic cytokine which stimulates melanin synthesis and prompts the expression of Microphthalmia-associated transcription factor (MITF) and tyrosinase in melanocytes.9
Since 1997, NB-UVB has been introduced as a clinically effective and safe tool in vitiligo management program, in which it stabilizes the depigmentation process and stimulates the residual follicular melanocytes to proliferate, produce melanin and migrate towards the adjacent depigmented skin.10,11 However, the molecular mechanism by which NBUVB induced pigmentation in vitiligo is still unclear. UVB may trigger particular chromophores in epidermal keratinocytes, melanocytes and dermal fibroblasts.12 Keratino-cytes may be stimulated to release unidentified cytokines and factors, which proposes that UVB functions as an immunomodulator.13
Yet, the role of LGI3 in the vitiligo pathogenesis was not investigated, as well the mechanism of NB-UVB induced pigmentation is quiet a substance of discussion and need to be elucidated. Therefore, the aim of the current study is to shed light on the hypothesized role of LGI3 in vitiligo development and to investigate if it has a role in NB-UVB induced pigmentation in vitiligo cases via evaluation of its immunohistochemical expression in lesional skin of vitiligo patients before and after NBUVB phototherapy sessions. Also, our aim was extended to assess whether there is any relationship between LGI3 immunohistochemical expression and the expected improvement of pigmentation in those patients.
MethodsForty patients with different degrees of vitiligo severity were included in this case-control study. They were recruited from the Dermatology Outpatient Clinic, Faculty of Medicine-Menoufia University Hospital, Shebin ElKom, Egypt. To limit the effects of the possible seasonal variation on the LGI3 secreted by keratinocytes, our patients were recruited during spring, from March to June 2016.
Additionally, 20 apparently healthy and vitiligo-free, age, gender, occupation and Fitzpatrick skin phototype-matched volunteers, with no family history of vitiligo, were included as a control group. The enrolled patients were instructed to stop treatment of their vitiligo 1 month prior joining the study. Patients having any skin disease other than vitiligo or those having history of photosensitivity to ultraviolet rays were excluded from the study. Each participant in the study signed a written consent form approved by the Research of Ethics committee in Human rights of Menoufia University, that was in accordance with the 1975 Helsinki Declaration (revised in 2000). The approval number of Research Ethics Committee for this study is 1105/10/6/2016.
A desired proposal sheet and general examination were performed to detect any excluding factor. Dermatologically, the studied subjects were evaluated to assess their skin phototype, and to classify vitiligo patients into segmental and non-segmental type.14,15 Vitiligo Area Severity Index (VASI) was calculated in the first visit and at week 12 (end of therapy) by the same physician.16
NB-UVB phototherapy was administered with the Waldmann UV 1000 L (TL 01) machine for the studied vitiligo cases three times/week on non-consecutive days for 12 weeks. We started the irradiation at 300 mj/cm2 (the minimum erythema dose of Egyptian skin).17 Then the dose was increased by 20% on each subsequent session till just faint erythema appeared. If symptomatic erythema (burning) or blisters developed, phototherapy was discontinued until the lesions healed. At that time, irradiation was restarted at a dose 20% lower. Thereafter the dose was increased by 10% on subsequent sessions.18 During treatment, genitals were shielded and eyes were protected with UV safety glasses.
From each patient, two punch skin biopsies from the involved skin were taken under local anesthesia, one before and one after NB-UVB phototherapy. In addition, one site-matched skin biopsy from every control subject was obtained. As controls did not undergo phototherapy, consequently no second biopsy was taken. All biopsies were submitted to the Pathology Department, Faculty of Medicine, Menoufia University. Sections were cut from the paraffin-embedded blocks and were stained with purified rabbit polyclonal antibody from Abcam (Cat.# ab113950) raised against LGI3, which was received as concentrated 0.1mL. The optimal dilution was 1:200, by using phosphate buffered solution (PBS). Negative control slides were prepared, by omitting the primary antibody from the staining procedure. Tissue sections prepared from human kidney tissue was used as a positive control for LGI3. Procedure of IHC staining was done according to received datasheet of the used antibodies.
Immunohistochemically, LGI3 expression is confirmed by cytoplasmic and/or membranous staining according to supplier’s datasheet, and was evaluated in both epidermis and dermis. In case of positively expressed cells, the percentage of the positive cell were assessed at x200 magnification field.19 Intensity of the stain was graded as mild, moderate or strong. Histo-score (H score) was calculated (H score = 1 × % of mildly stained cells + 2 × % moderately stained cells + 3 × % of strongly stained cells).20 LGI3 stain distribution pattern was categorized as either patchy or diffuse and its cellular localization was assigned as either cytoplasmic and/or membranous.
Statistical analysisThe results were collected, entered and processed on IBM-PC compatible computer using SPSS software (version 20.0) (SPSS Inc., Chicago, U.S). Two types of statistics were done: descriptive statistics [e.g. percentage (%), mean (×) and standard deviation (SD)], and analytic statistics, which included: Student’s t and Mann Whitney U test to compare quantitative data according to its distribution. Fisher’s Exact test was used in the analysis of 2×2 contingency tables when at least 25% of cells has expected number < 5. Marginal Homogeneity test (MH) was used on paired (e.g. pre and post) qualitative data when a category of the sample is more than two. Chi-square test was used for qualitative data and Spearman correlation to assess correlation. P <0.05 was considered the cut-off value for significance.
ResultsClinical data of the studied groups:
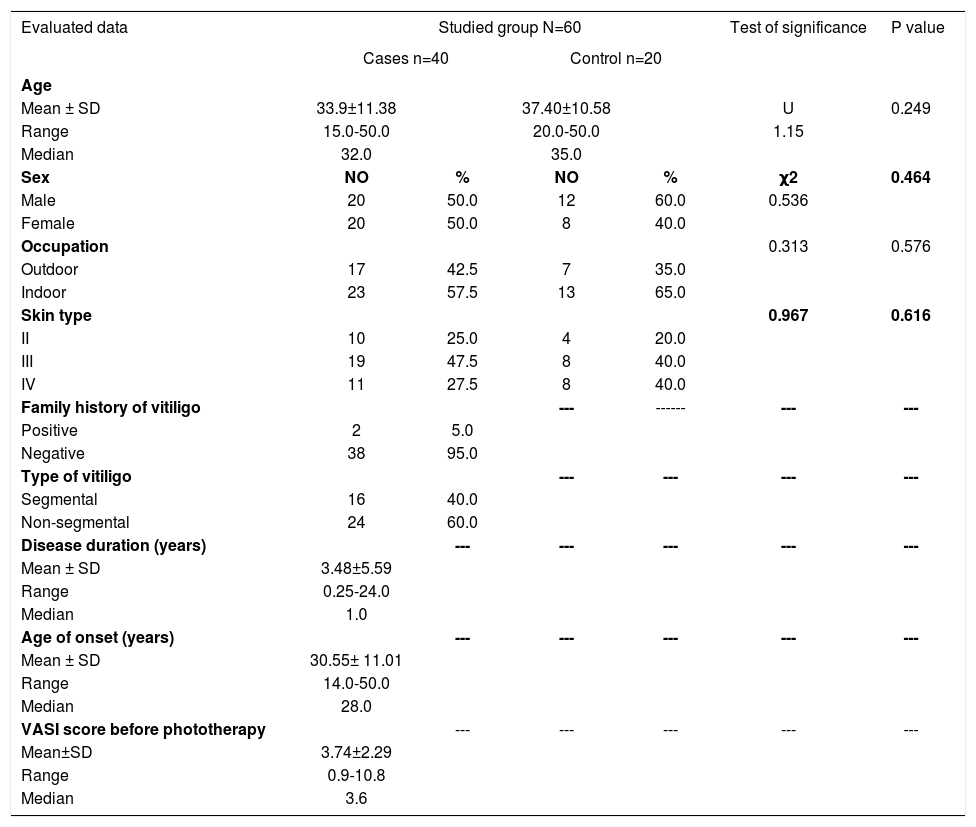
Clinical data describing patients’ demographics as well as the clinical variables were obtained and are documented in table 1.
Clinical data of the studied groups
| Evaluated data | Studied group N=60 | Test of significance | P value | |||
|---|---|---|---|---|---|---|
| Cases n=40 | Control n=20 | |||||
| Age | ||||||
| Mean ± SD | 33.9±11.38 | 37.40±10.58 | U | 0.249 | ||
| Range | 15.0-50.0 | 20.0-50.0 | 1.15 | |||
| Median | 32.0 | 35.0 | ||||
| Sex | NO | % | NO | % | χ2 | 0.464 |
| Male | 20 | 50.0 | 12 | 60.0 | 0.536 | |
| Female | 20 | 50.0 | 8 | 40.0 | ||
| Occupation | 0.313 | 0.576 | ||||
| Outdoor | 17 | 42.5 | 7 | 35.0 | ||
| Indoor | 23 | 57.5 | 13 | 65.0 | ||
| Skin type | 0.967 | 0.616 | ||||
| II | 10 | 25.0 | 4 | 20.0 | ||
| III | 19 | 47.5 | 8 | 40.0 | ||
| IV | 11 | 27.5 | 8 | 40.0 | ||
| Family history of vitiligo | --- | ------ | --- | --- | ||
| Positive | 2 | 5.0 | ||||
| Negative | 38 | 95.0 | ||||
| Type of vitiligo | --- | --- | --- | --- | ||
| Segmental | 16 | 40.0 | ||||
| Non-segmental | 24 | 60.0 | ||||
| Disease duration (years) | --- | --- | --- | --- | --- | |
| Mean ± SD | 3.48±5.59 | |||||
| Range | 0.25-24.0 | |||||
| Median | 1.0 | |||||
| Age of onset (years) | --- | --- | --- | --- | --- | |
| Mean ± SD | 30.55± 11.01 | |||||
| Range | 14.0-50.0 | |||||
| Median | 28.0 | |||||
| VASI score before phototherapy | --- | --- | --- | --- | --- | |
| Mean±SD | 3.74±2.29 | |||||
| Range | 0.9-10.8 | |||||
| Median | 3.6 | |||||
-VASI: Vitiligo Area Severity Index -U: Man-Whitney test -χ2: Chi-Square test
Baseline LGI3 immunohistochemical staining in the studied groups:
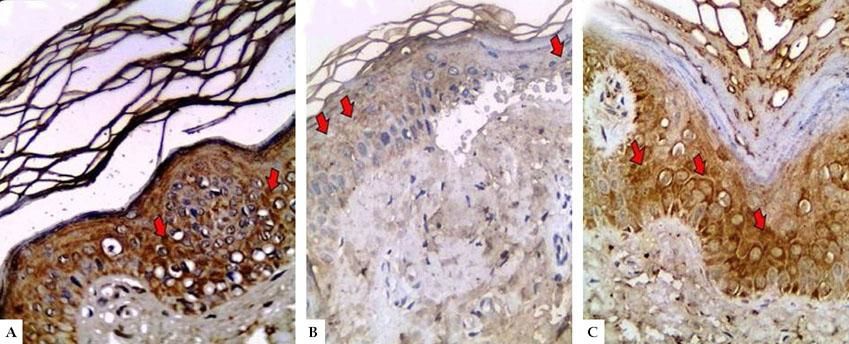
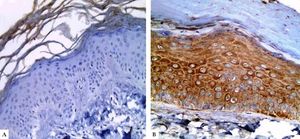
In controls, LGI3 expression was positive in all sections (100%), was mainly of cytoplasmic localization (18, 90%), and showed mild intensity in half of evaluated tissues (50%), with patchy distribution in 14 sections (70%) (Figure 1A). The percentage of LGI3 immunoreactivity ranged from 10 to 85 with a mean of 40.25 ± 26.83, and its H score ranged from 10 to 255 with a mean of 89.75 ± 94.79.
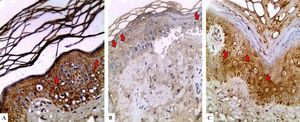
LGI3 immunohistochemical expression in A. normal skin biopsy shows diffuse strong cytoplasmic expression (red arrows), B. vitiligo case before NB-UVB phototherapy, clearly shows minimal mild expression (red arrows), and C. the same vitiligo case shows increased expression of LGI3 after NB-UVB phototherapy (red arrows) (immunoperoxidase x400HPF)
However, in vitiligo patients, LGI3 immunohistochemistry before phototherapy showed that LGI3 expression was positive in 27 patients (67.5%), most of which were of cytoplasmic localization (24, 88.9%), and revealed mild intensity (22, 81.5%) (Figure 1B). Additionally, patchy distribution was observed in 21 sections (77.8%). The percentage of LGI3 immunoreactivity ranged from 2 to 70 with a mean of 18.22 ± 18.44, and its H score ranged from 2 to 120 with a mean of 25.26 ± 31.03.
There was significant reduction in LGI3 immunohistochemical staining in vitiligo patients compared to controls regarding LGI3 expression (67.5% vs 100%) (P=0.003) and intensity, that was mainly mild in vitiligo cases (81.5% VS 50%) (P=0.001). Moreover, LGI3 expression percentage and H score were significantly lower in vitiligo patients than in controls (18.22±18.44 VS 40.25±26.83 and 25.26± 31.03 vs 89.75±94.79, respectively) (P =0.001 for both) (Table 2).
LGI3 immunohistochemical staining of vitiligo patients before and after phototherapy, and controls
| Immunohis-tochemical staining | Studied group (N=60) | Test of Significance | p-value | |||||
|---|---|---|---|---|---|---|---|---|
| Controls n=20 | Cases before treatment n=40 | Cases after treatment n=40 | ||||||
| Expression | NO | % | NO | % | NO | % | T1=χ2=8.29 | P1=0.003* |
| Positive | 20 | 100 | 27 | 67.5 | 37 | 92.5 | T2=Mc Nemar χ2=10.58 | P2=0.001* |
| Negative | 0 | 0.0 | 13 | 32.5 | 3 | 7.5 | T3= χ2=1.85 | P3=0.554 |
| Localization | ||||||||
| Cytoplasmic | 18 | 90 | 24 | 88.9 | 31 | 83.8 | T1= χ2=0.83 | P1=0.659 |
| Membranous | 0 | 0 | 1 | 3.7 | 1 | 2.7 | T2=MH=0.447 | P2=0.655 |
| Membrano-cytoplasmic | 2 | 10 | 2 | 7.4 | 5 | 13.5 | T3= χ2=0.73 | P3=0.694 |
| Intensity | ||||||||
| Mild | 10 | 50.0 | 22 | 81.5 | 18 | 48.6 | T1= χ2=8.65 | P1=0.013* |
| Moderate | 5 | 25.0 | 5 | 18.5 | 14 | 37.8 | T2=MH=2.99 | P2=0.003* |
| Strong | 5 | 25.0 | 0 | 0.0 | 5 | 13.5 | T3= χ21.62 | P3=0.444 |
| Distribution | T1= χ2=0.366 | P1=0.545 | ||||||
| Patchy | 14 | 70.0 | 21 | 77.8 | 20 | 54.05 | T2=Mc Nemar χ2 =6.50 | P2=0.011* |
| Diffuse | 6 | 30.0 | 6 | 22.2 | 17 | 45.9 | T3= χ2=1.37 | P3=0.242 |
| Percentage | ||||||||
| Mean ± SD | 40.25±26.83 | 18.22±18.44 | 39.07±24.89 | T1=U=116.00 | P1=0.001* | |||
| Range | 10.0-85.0 | 2.0-70.0 | 2.0-95.0 | T2=Wilcoxon signed rank=4.29 | P2=0.001* | |||
| Median | 30.0 | 10.0 | 30.0 | T3=U=0.528 | P3=0.597 | |||
| H score | ||||||||
| Mean ± SD | 89.75±94.79 | 25.26±31.03 | 74.11±71.96 | T1=U=3.39 | P1=0.001* | |||
| Range | 10.0-255.0 | 2.0-120.0 | 2.0-240.0 | T2=Wilcoxon signed rank =4.38 | P2=0.001* | |||
| Median | 40.0 | 10.0 | 45.0 | T3=U=0.570 | P3=0.569 | |||
T1: test of significance for comparison between cases before treatment and controls; T2: test of significance for comparison between cases before and after treatment; T3: test of significance for comparison between cases after treatment and controls; P1: p-value of comparison between cases before treatment and controls; P2: p-value of comparison between cases before and after treatment; P3: p-value of comparison between cases after treatment and controls; *: Significant.
Results after phototherapy:
LGI3 immunohistochemical expression in vitiligo patients:
After 12 weeks of NB-UVB phototherapy, LGI3 immunohistochemical staining demonstrated significant increase compared to before phototherapy (37, 92.5% vs 27, 76.5%) (P=0.001) (Figure 1C, Figure 2). Also, its intensity was significantly intensified from mild (22, 81%) in pretreatment to moderate and strong (19, 51.3%) in post treatment biopsies (P=0.003). Furthermore, LGI3 expression percentage and H score revealed also significant increase in post treatment compared to pretreatment sections (39.07±24.89 vs 18.22±18.44 and 74.11±71.96 vs 25.26±31.02 respectively) (P =0.001 for both) (Table 2).
The observed up-regulated LGI3 immunohistochemical staining parameters in vitiligo patients after phototherapy matched that of controls (p > 0.05 for all) (Table 2).
VASI score in vitiligo patients:
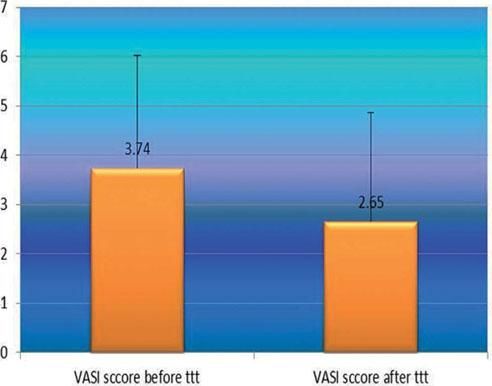
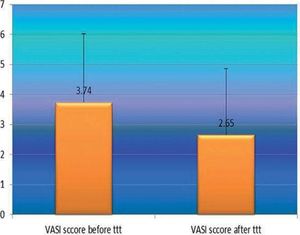
After phototherapy, there was an observed clinical improvement in studied vitiligo patients and a significant decrease in their VASI score mean values compared to before phototherapy (2.65 ± 2.22 vs 3.34 ± 2.29) (P=0.001) (Figure 3).
Relationship between VASI score improvement and the studied clinical parameters of vitiligo patients:
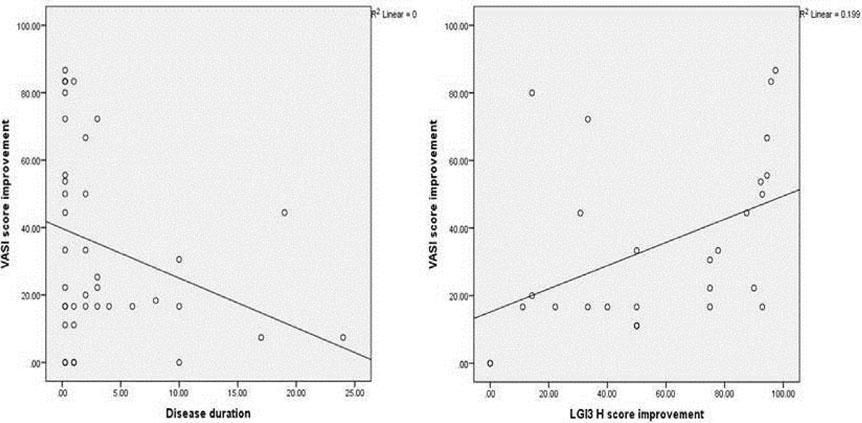
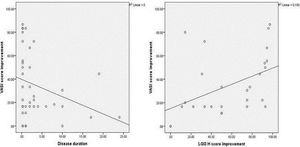
The improvement in VASI score was not significantly associated with any of the studied parameters, except for disease duration which showed a significant negative correlation (r= - 0.349, P = 0.027) (Figure 4A).
Correlations between the improvement in VASI score and the improvement in LGI3 H score among vitiligo patients:
There was a significant positive correlation between improvement in VASI score and improvement in LGI3 H score mean values in the vitiligo cases studied (r=0.560, P=0.002) (Figure 4B).
DiscussionAs far as we know, this is the first study to investigate the role of LGI3 in vitiligo, from which we suggested the important role of LGI3 down regulation in vitiligo pathogenesis and its active role in NB-UVB induced pigmentation in vitiligo cases.
In the present study, LGI3 immunohistochemical staining of control subjects showed positive immune-reactivity in all examined sections (100%), that was of cytoplasmic localization in most of the evaluated tissues (90%). In line with this result, Lee et al. (2012) revealed for the first time that LGI3 is expressed mostly by normal human keratinocytes. Moreover, the authors reported that LGI3 motivates the phosphorylation of a serine/threonine kinase (Akt), which is convoluted in the cell survival-signaling cascade. Furthermore, LGI3 stimulates the phosphorylation of Mouse double minute 2 homolog (MDM2) and consequent P53 degradation. Taken together, the authors suggested that LGI3 may adjust P53 level and that keratinocyte-derived LGI3 may function as a unique cytokine for skin homoeostasis.6
Significant down regulation of LGI3 protein in vitiligo patients in comparison to their matched peers was displayed in the current study. Supporting our findings, Jeong and his co-workers examined the expression and distribution of LGI3 in various skin tissues. The authors carried out immunohistochemical and Fontana-Masson staining using normal, hypopigmented (vitiligo) and hyperpigmented (melasma) human skin tissue samples (2 cases in each). They observed higher expression of LGI3 in the skin with melasma, while skin with vitiligo exhibited lower expression of LGI3 compared to control sections.9 Therefore, we may hypothesize that LGI3 participates in melanogenesis as a keratinocyte-derived factor in a paracrine manner.
Confirming our postulation, Jeong et al. investigated the effects of LGI3 on melanin synthesis. They treated normal human melanocytes and Mel-Ab cells with 0-10ng/ml recombinant LGI3 for 4 and 5 days respectively. After that, these treated cells were photographed under a phase contrast microscopy and their protein contents was quantified. The authors demonstrated that LGI3 promoted melanin synthesis in both cell types. Moreover, they observed up-regulation of MITF and tyrosinase enzyme at both protein and mRNA levels by means of Western blotting and RT-PCR respectively.9
Tyrosinase, a copper-containing oxidase, is the rate-limiting enzyme for regulation of melanin production. It is mainly involved in the hydroxylation of a monophenol and in the transformation of an o-diphenol to its analogous o-quinone. O-Quinone undergoes several reactions and finally forms melanin.21 MITF, a basic helix-loop-helix-leucine-zipper transcription protein, plays an essential role in the development of numerous cell types from the neural crest including melanocytes, in which it controls the expression of several genes that are necessary for normal synthesis of melanin. Moreover, MITF has been implicated in cell cycle regulation and plays a vital role in self-renewal and upkeep of melanocyte stem cells.22
After NB-UVB phototherapy sessions, in agreement with previous studies, we observed significant clinical improvement that was pronounced in patients with recent vitiligo compared to those with long standing disease.17,23,24
Parallel to the clinical improvement, LGI3 immunoreactivity in vitiligo patients showed significant up-regulation after NB-UVB phototherapy in all of its evaluated parameters, and became comparable to that of controls. Supporting our result, Lee et al. reported that LGI3 expression was increased in all layers of the rat epidermis after UVB irradiation. Furthermore, ELISA analysis showed that HaCaT human keratinocytes increased LGI3 secretion after exposure to UVB in a time and dose-dependent manner.6
Moreover, in the current study, the improvement in LGI3 H score was significantly correlated with the degree of observed repigmentation indicated by the improved VASI score. So, we may suggest the possible active role of LGI3 in repigmentation process induced by NB-UVB phototherapy sessions.
Melanogenesis is the procedure of melanin synthesis through keratinocytes-melanocytes collaboration, which is triggered by ultraviolet-B damaging effect with consequent skin and hair pigmentation. After the skin is exposed to UVB irradiation, keratinocytes intensely synthesize numerous biochemical factors such as alpha-Melanocyte-stimulating hormone (a-MSH), stem cell factor (SCF), endothelin-1 (ET-1) and prostaglandin E2 (PGE2). These keratinocytes-derived factors are transferred to melanocytes in a paracrine way and activate MITF through a sequence of signaling events, which results in melanin formation through stimulation of tyrosinase-related proteins. Synthesized melanin is then transmitted to the surrounding keratinocytes to protect cells from UVB-in-duced DNA injury.25
Herein, we suggest that LGI3 secreted by NB-UVB irradiated keratinocytes may have a stimulatory effect on melanocytes and may actively participate in NB-UVB induced pigmentation in vitiligo patients. This effect may be mediated through up-regulation of MITF, tyrosinase, and/or induction of β-catenin buildup through the Akt pathway and regulation of skin homeostatic procedures via the Wnt/β-catenin pathway.9,26
Like MITF, the Wnt/β-catenin signaling pathway has been concerned in melanocyte development and survival. Furthermore, it was found that β-catenin functionally interacts with the MITF protein itself and inhibition of β-catenin can effectively reduce the melanin production.27
Relatively small sample size was the main limitation of the current work. Therefore, we recommend further large-scaled studies to validate our findings as this is the first study that evaluates LGI3 protein expression in vitiligo patients.
ConclusionBased on our result, we concluded that reduced LGI3 protein may have a role in vitiligo etiopathogenesis and its up-regulation after NB-UVB phototherapy may actively contribute in NB-UVB photo-induced melanogenesis. Moreover, from this piece of work, LGI3 may open the door for a novel therapeutic option in the vitiligo management program.
AcknowledgementThe authors are grateful to administrative and technical staffs at the Dermatology Clinic and Pathology Department, Faculty of Medicine, Menoufia University, Egypt, who kindly helped throughout this study.