Dear Editor,
Systemic lupus erythematosus (SLE) is an autoimmune disease with multiple clinical manifestations, such as renal, hematological, neurological, and cutaneous. Acute cutaneous lupus erythematosus (ACLE), subacute cutaneous lupus erythematosus (SCLE), and chronic cutaneous lupus erythematosus (CCLE), which includes discoid lupus erythematosus (DLE) and lupus erythematous panniculitis, are among the classic skin manifestations, present in 80% of SLE cases.
Photoprotection, antimalarials, dapsone, thalidomide, and corticosteroids are the treatments of choice for cutaneous lupus erythematosus (CLE). Immunosuppressive drugs, such as azathioprine, methotrexate, and cyclosporine, may also be used to control the disease. However, some cases are refractory to such treatments. The anti-CD20 monoclonal antibody rituximab (RTX) has been used as a therapeutic option in refractory cases with good results, mainly in patients with SCLE and severe extracutaneous forms of SLE.1
We aim to demonstrate a case of SCLE, refractory to conventional therapy, that was successfully treated with RTX and to review the literature of SCLE cases treated with RTX.
A 31-year-old female, brown-skinned, hypertensive and smoker, has been attending our outpatient clinic since 2002 due to SLE. She presents SCLE, photosensitivity, lymphopenia (456/ml), antinuclear antibodies (ANA) 1/640 with a homogenous pattern, and other immunological alterations (anti-Sm, anti-RNP, anti-Ro). No signs of renal, neurological or pulmonary impairment have been detected.
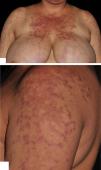
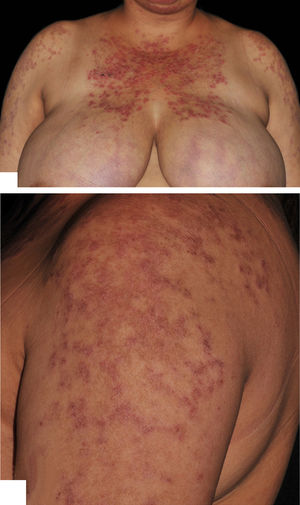
Physical examination revealed violaceous, erythematous, annular, exulcerated, and scaly plaques, mainly on sun-exposed areas, and labial mucous damage with exulcerated plaques (Figure 1).
The patient had previously been treated with azathioprine (150mg/day), hydroxychloroquine (400mg/day), thalidomide (100mg/day), dapsone (100mg/day), prednisone (0.5 to 1mg/kg/day), and monthly pulse therapy with methylprednisolone (21 cycles) without complete control of the disease.
As an attempt of treatment, RTX was started at a weekly dose of 375mg/m2 for 4 weeks, followed by maintenance doses every 6 months. Intravenous methylprednisolone (100mg) was also given with each RTX dose. One month after the first dose, the patient showed significant improvement with a complete and continuous control of the disease, reducing her disease activity index (SLEDAI) from 18 to 4.
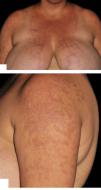
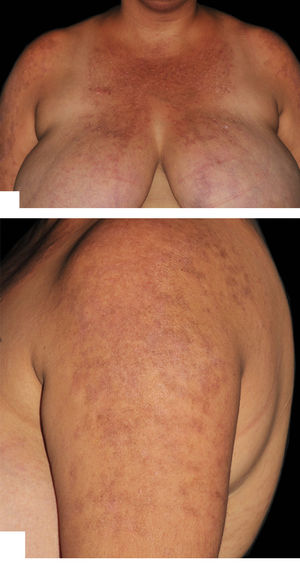
Hydroxychloroquine, thalidomide, and dapsone doses were maintained and oral prednisone was gradually tapered without flares throughout a 12-month follow-up (Figure 2).
RTX is an anti-CD20 monoclonal antibody approved for treatment of B-cell lymphomas and rheumatoid arthritis. However, studies have shown its effectiveness in treating autoimmune skin disorders, such as pemphigus vulgaris, pemphigus foliaceus, vasculitis, and SLE, specially SCLE. RTX is an immunosuppressive drug that enables the gradual tapering of oral corticosteroids, thus reducing the side effects caused by its chronic use.2
Currently, there are only eight published cases of patients with refractory SCLE treated with RTX (Table 1).2-5 In all published cases, the SCLE patients selected to use RTX had otherwise been refractory to conventional therapy. Except for 1 case, the remaining 7 patients showed effective results with little side effects.
Review of published cases of subacute cutaneous lupus erythematosus treated with rituximab (RTX)
| Sex/age | Therapy scheme | Therapeutic response | Maintenance therapy | Relapse | Previous therapy |
|---|---|---|---|---|---|
| F/402 | 2x 1g RTX + 100mg IV methylprednisolone (every 2 weeks) + 750mg of cyclosporine after first infusion | Little | Not specified | None | HCQ, Pred, MTX, MMF |
| F/742 | 2x 1g RTX + 100mg IV methylprednisolone (every 2 weeks) + 750mg of cyclosporine after first infusion | remission | Not specified | None | Pred, Aza, MTX |
| F/652 | 2x 1g RTX + 100mg IV methylprednisolone (every 2 weeks) + 750mg of cyclosporine after first infusion | Complete remission | Not specified | None | Pred, MMF |
| M/443 | 2x 1g RTX (weekly dose) | Complete remission | None | None | Pred, MMF, HCQ, Aza |
| F/484 | 4x 375mg/m2 RTX (weekly dose) | Complete remission | 1 dose every 8 weeks for two years | 11 months after first dose | HCQ, Pred, MTX, dapsone, Aza |
| F/545 | 4x 375mg/m2 RTX (weekly dose) | Complete remission | 2x 1g every 2 weeks for one year | None | HCQ, Pred, MMF, thalidomide, IGIV, etanercept |
| F/375 | 4x 375mg/m2 RTX (weekly dose) | Complete remission | 2x 1g every 2 weeks for one year | None | HCQ, Pred, Aza |
| F/285 | 4x 375mg/m2 RTX (weekly dose) | Partial remission | Not specified | None | HCQ, Pred |
RTX: rituximab/ Pred: prednisone/ Aza: azathioprine/ MMF: mycophenolate mofetil/ HCQ: Hydroxychloroquine/ MTX: methotrexate / IGIV: intravenous immunoglobulins/ F: female/ M: male
RTX doses varied from 1g twice a week to 375mg/m2 a week for 4 weeks. Maintenance doses, when used, varied from 2 months to 1 year. In the case we report here, the dose was similar to that used in the Australian study (weekly doses of 375mg/m2 for 4 weeks). However, since the patient’s condition improved significantly and there were no flares, maintenance doses were used every 6 months.4
Similar to most cases reported in the literature, our patient’s condition improved one month after the first RTX dose, which enabled the gradual tapering of prednisone, thus reducing the side effects caused by its chronic use. Some studies reported flares after treatment started, but lesions were cleared after maintenance doses.
Although RTX has shown effective results in the treatment of SCLE, it has not been effective for cutaneous lesions of other forms of CLE, such as CCLE. This suggests that RTX is indicated for lupus manifestations involving Th2 cells, such as serositis, vasculitis, nephritis, ACLE and SCLE, which are mediated by B cells (CD20+).
Further studies are needed in order to recognize RTX as a therapeutic option for SCLE, even though independent results indicate that it is effective in the treatment of refractory SCLE. Data regarding dose / frequency of administration, drug tolerability, side effects, and long-term remission are still lacking given the small number of reported cases.
Financial support: None.
Conflict of interests: None.