This study aims to evaluate tissue healing efficacy in burn patients treated with 1% silver sulfadiazine versus other treatments.
This is a systematic literature review and meta-analysis of randomized clinical trials performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyzes (PRISMA) and PICO strategy, registered in the International Prospective Register of Systematic Reviews (PROSPERO) under the number CRD42017081057.
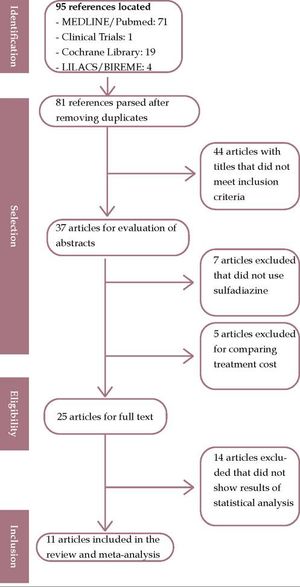
The review found 71 studies in MEDLINE/Pubmed, 1 in Clinical Trials, 19 in the Cochrane Library, and 4 in LILACS in five manual searches. Of these, 81 studies were pre-selected. After independent analysis by two reviewers, only 11 studies met the inclusion criteria for the review.
All studies (n = 11) using alternative treatments to silver sulfadiazine were shown to be superior in the mean time for complete wound healing, with statistically significant differences between experimental and control groups (p <0.00001); mean difference (-4.26), 95% CI [- 5.96, -2.56].
Burns are a major public health problem even today. Such injuries can be caused by various agents, including thermal energy, electric shock, chemicals, radiation, and movement causing abrasion. These factors produce excessive heat, which damages the body tissues and causes cellular degradation, generating tissue death, with skin as the main organ affected.1 Burns can be classified in three types, according to degree of severity and tissue damage: small, medium, and large and first, second, and third-degree, re-spectively.1 Most burns happen in the home environment. These accidents involve huge hospital expenses and leave physical and psychological sequelae.1 Brazil reported 26,683 cases of burns and corrosion injuries in 2016 and 2017. Of these, 84.9% required emergency treatment and 28.3% occurred in children <1 to 9 years old.2 The North of Brazil was the region that reported the fewest burns, with approximately 5.8% of hospital admissions according to data from 2016 and 2017.2 The Southeast region had the most hospitalizations due to burns, with 9,094 admissions in 2016 and 2017.2 Burn treatment aims to promote complete and rapid healing of wounds, reduce pain, protect the wound, prevent infection, and reduce physical sequelae and functional disability.3 For decades, routine management has centered on ointments and dressings containing 1% silver sulfadiazine (SSD) as the gold standard treatment for burns, due to the drug’s antimicrobial properties against a wide range of gram-positive and gram-negative microorganisms.3 However, like all drugs, SSD has some undesirable effects, such as decrease in wound contraction, black scars, delayed development of granulation tissue, limited depth of penetration in lesions, risk of silver toxicity, and neutropenia.4 Recent studies have shown that certain agents used in the past, such as SSD, are no longer as effective in inhibiting bacterial growth in vitro, mainly due to cases of multire-sistant bacteria.5 Thus, numerous attempts have been made to find alternative dressings that offer better healing efficacy as well as tolerability. The results are still conflicting as to the real existence of other products that may offer better healing outcomes than SSD.6 This study thus aims to evaluate healing efficacy in burn patients treated with silver sulfadiazine 1% versus other treatments.
MethodsThis is a systematic literature review and meta-analysis performed with the PICO strategy, where P = burn patients hospitalized in the burn ward; I = new treatments; C = silver sulfadiazine; O = complete healing. This study was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Me-ta-Analyses (PRISMA), a guide for good research practices. A research protocol was created to guide the authors in all the review stages and was registered in the International Prospective Register of Systematic Reviews (PROSPERO), an international virtual database for registration and monitoring of systematic reviews, under the number CRD42017081057.
Eligibility criteriaThe eligibility criteria for studies were: (1) randomized controlled trials, open or blinded; (2) studies demonstrating the use of silver sulfadiazine as the gold standard for the treatment of burns; (3) data available to measure tissue healing efficacy by follow-up time; and (4) no restriction as to age or sex.
Data sourcesAn electronic search was performed for articles published until August 2017 in MEDLINE/Pubmed, Clinical Trials, LILACS/BIREME, and Cochrane Library, with no restriction on language or year of publication.
Search strategyThe search strategy in MEDLINE/Pubmed used the following keywords: (“silver sulfadiazine” and “burns” [Mesh]) AND “wounds and injuries” Mesh]) AND “randomized controlled trial” [OR] “random”, adapted to the other databases when necessary.
Selection of studiesThe eligibility criteria were applied to all titles and abstracts by two independent reviewers (ABSM, JFO). In case of disagreement between the reviewers, a third reviewer was consulted.
Measures-SummaryThe principal measure of therapeutic efficacy was the difference in tissue healing time between the experimental and control groups.
Methodological Quality AssessmentIn the evaluation of methodological quality of the selected articles, the Jadad scale was used, which evaluates and highlights five specific topics: (1) Was the study described as randomized? (2) Was the randomization method appropriate? (3) Was the study described as double-blind? (4) Was the blinding method appropriate? (5) Was there a description of exclusions and losses? Thus, the quality of the selected studies can be evaluated with conceptual scores: 1 (low quality); 2-3 (average quality); 4-5 (high quality)
Data extractionData from selected articles were extracted by two independent researchers (BSS, ABSM) for a preestablished record. The authors were blinded as to the abstracts to decrease selection bias. Items were collected according to the following: sample characteristics, experimental group’s characteristics (treatments used, doses, duration of interventions, and dose intervals), and methodological quality. Mean complete healing time in the experimental and control groups was defined as the primary endpoint to test the interventions’ efficacy. Other observations such as infections, bacterial colonization, presence of exudate, inflammation, and pain were described as the interventions’ malfunctions, when present.
Data analysisData were entered into a Microsoft Office Excel (2007) spreadsheet with essential items such as bibliographic data, title, objectives, study design, context, participants, year of data collection, variables, sample size, statistical methods, results, tables, and graphs. Regarding the metanalysis, data were keyed in and analyzed in Review Manager (RevMan), version 5.3. Data were classified as continuous, and means and standard deviations were calculated, as well as total of individuals belonging to the experimental and control groups, and converted into mean differences (treatment effect) with 95% confidence interval (95% CI). The random effects model was used due to heterogeneity between the studies, providing more conservative estimates of the treatment effects. The studies’ statistical heterogeneity was calculated with the chi-square test, with p-value <0.1 considered significant, and the I2 statistic with values > 50%. Publication bias was evaluated by funnel plot and Egger regression test.
ResultsA total of 95 primary studies were identified as results: (n = 71) on MEDLINE / PubMed, (n = 1) in Clinical Trials, (n = 19) in Cochrane Library, and (n = 4) in LILACS in five manual searches. Of these, 81 studies were pre-selected. Figure 1 shows that after independent analysis by two reviewers, only 11 studies met the inclusion criteria and were thus included in this review. A third reviewer’s opinion was solicited for consensus on 11 articles selected qualification.
Table 1 presents the selected studies’ synthesis and scores obtained in methodological quality evaluation by the Jadad scale.7-17 Studies were included with the year of publication from 2007 to 2017. Of the 11 eligible studies, 3 (27.2%) were conducted in Thailand, 3 in Iran, 2 in Pakistan, 2 in China, and 1 in Germany. All the studies were randomized, totaling 755 patients treated in hospitals with wards for burn patients.
Selected studies, clinical trial synthesis, and scores obtained in methodological quality evaluation according to the Jadad scale
| Study | Study Type (n) Patients | Intervention | Follow-up time | Follow-up time | Jadad Scale |
|---|---|---|---|---|---|
| Genuíno, 20147 | Randomized Clinical Trial (n = 50) | Experimental: Petrolatum gel Control: SSD | Until the end | GE: 6.2 ± (SD 2.8) GC: 7.8 ± (SD 2.1) (p = 0.050) | 3 |
| Saeidinia, 20178 | Randomized Clinical Trial (n = 75) | Experimental: Centiderm Control: SSD | 25 days | GE: 14.67± (SD 1.78) CG: 21.53 ± (SD 1.65) (P = 0.001) | 5 |
| Shahzad, 20139 | Randomized Clinical Trial (n = 50) | Experimental: Aloe vera Gel Control: SSD | 60 days | GE: 11 ± (SD 4.18) CG: 24.24 ± (SD 11.16) (p = < 0.0001) | 1 |
| Nasiri, 201610 | Randomized Clinical Trial (n = 90) | Experimental: Arnebia euchro-ma ointment Control: SSD | 30 days | GE: 13.9 ± (SD 5.3) GC: 17.5 ± (SD 6.9) (p =0.001) | 4 |
| Muangman, 201011 | Randomized Clinical Trial (n = 70) | Experimental: Aquacel® Ag dressing Control SSD | 35 days | GE: 10 ± (SD 3.0) GC: 13.7 ± (SD 4.3) (p = 0.02) | 2 |
| Malik, 201012 | Randomized Clinical Trial (n = 150) | Experimental: Honey Control SSD | Until the end | GE: 13.47 ± (SD 4.0) GC: 15.62 ± (SD 4.40) (p < 0.0001) | 1 |
| Homann, 200713 | Randomized Clinical Trial (n = 47) | Experimental: Liposome Hy-drogel with Polyvinyl-Pyrrolidone Iodine Control: SSD | Until the end | GE: 9.9 ± (SD 4.5) GC: 11.3 ± (SD 4.9) (p = 0.015) | 2 |
| Huang, 200714 | Randomized Clinical Trial (n = 98) | Experimental: Acticoat Control: SSD | 20 days | GE: 12.42 ± (SD 5.40) GC: 15.79 ± (SD 5.60) (p = 0.005) | 3 |
| Aramwit, 201315 | Randomized Clinical Trial (n = 29) | Experimental: Sulfadiazine Silk Sericin Control: SSD | Until the end | GE: 22.42 ± (SD 6.33) GC: 29.28 ± (SD 9.27) (p = 0.001) | 5 |
| Maghsoudi, 201316 | Randomized Clinical Trial (n = 50) | Experimental: Platelet Healing Control: SSD | Until the end | GE: 9.5 ± (SD 4.6) GC: 12.2 ± (SD 5.4) (p < 0,0001) | 3 |
| Wattanaploy,201717 | Randomized Clinical Trial (n = 46) | Experimental: Polyhexanide/Betaine Gel Control: SSD | 22 days | GE: 17.8 ± (SD 2.2) GC: 18.8 ± (SD 2.1) (p=0.13) | 2 |
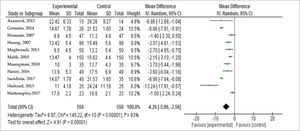
A meta-analysis was performed with the 11 studies that reported healing time for each group with means and standard deviations. For these data analyses, Review Manager version 5.3, available from the Cochrane Library, was used. Regarding healing outcome, using a random effects model, a statistically significant difference between experimental and control groups (p <0.00001) was evident, as shown in figure 2; mean difference - 4.26, 95% CI [-5.96, -2.56].
The studies’ heterogeneity showed chi-square = 145.22 (p <0.00001) and I2 statistic = 93%. Thus, although the studies had the same cause and effect, they were not conducted in the same way, having different and varied samples, leading to different observations. The random effects model was thus chosen to explain the results more comprehensively.
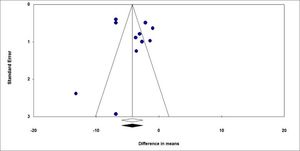
We performed a funnel chart to investigate for potential publication bias, as demonstrated below (Figure 3). The vertical axis represents study size (standard error), while the horizontal axis represents the effect size (logarithmic probabilities ratio). This graph shows a slight asymmetry sign to one side of the mean. However, the Egger regression test (intercept = 1.277) was not significant (p = 0.48), thus failing to detect a publication bias. The use of treatments other than silver sulfadiazine was significantly relevant in favor of experimental groups compared to control groups, favoring burn healing, as evidenced by the diamond in figure 2. Shahzad et al.9 reported different results from most of the other studies included in this meta-analysis, with a high standard deviation in the sulfa-diazine group compared to the intervention group (11.16 vs 4.18). This difference is due in part to inhomogeneity between groups and ineffective blinding and randomization. Other problems that may explain the difference include the drug dose, withdrawal time, and patient management by the various attending professionals. The studies by Shahzad et al.9 and Malik et al.12 were found to have high risk of bias because they were classified as low quality according to the Jadad scale (score 1), due in part to non-blinding of patients, failure to specify losses and exclusions, and ineffective randomization.
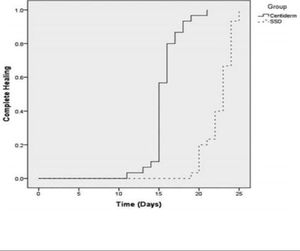
DiscussionRegarding the outcome “healing time”, the interventions in 100% of the studies (n = 11) proved superior to silver sulfadiazine in mean complete wound healing time. Five studies (45.4%) reported no patient follow-up, and the outcome was the end of observation. Mean follow-up time in the other studies was 32 ± 14.76 days. Saei-dinia8, Shahzad9, and Aramwit15 observed a larger mean difference in days of complete healing between the experimental and control groups: 14.67 vs 21.53; 11 vs 24.24, and 22.42 vs 29.28 respectively, using three experimental products: Centiderm, Aloe vera, and sulfadiazine with sericin. The clinical trial that found the best results in favor of experimental treatment was performed by Saeidinia et al.8, showing shorter healing time than in the other studies. The authors blinded the sample and randomized patients to compare two sulfadiazine-based products. The first was Centiderm, a herbal extract of Centella asiática species produced by pharmacognosy at Guilan Pharmacy Faculty, and the other was a conventional dressing with SSD. The sample had a total sample of 75 patients, randomized into two groups: (40 in the experimental arm and 35 controls, with a follow-up time of 25 days. The outcome is shown below (Figure 4). Average burn healing time was 14.67 ± 1.78 vs 21.53 ± 1.65 days. In the experimental group, 43.3% (n = 13) of the burns were caused by water vapor, and in the control group 53.3% (n = 16) by hot water.8
Kaplan-Meier curve for complete healing by follow-up time Source adapted from: Saeidinia et al.8
Wattanaploy et al.17 in 2017 randomized two groups of 23 patients each with a follow-up time of 22 days and compared sulfadiazine with a polyhexanide/betaine gel (Prontosan®), a surfactant that cleans the wound, controls exudate, and promotes debridement. There was no statistically significant difference between the groups (p = 0.13) in colonization of the wound with bacteria, with 26.1% (n = 6) in both groups.17 In 2013, Maghsoudi et al.16 in Iran investigated whether platelet-derived growth factor (PDGF), which increases the synthesis of collagen and proteoglycans, was also effective in reducing healing time in burns, since one of its PDGF-BB isomers had already proven effective in some randomized clinical trials in patients with diabetic foot. The authors compared a conventional silver sulfadiazine dressing with an experimental platelet concentrate made by the institution itself. Each patient received two treatments and was assessed at the end of follow-up. The authors found innovative results, with mean healing time of 9.5- + 4.6 days versus 12.2 +/- 5.4 in the silver sulfadiazine group (p <0.0001).16 In 2014, Genuino et al.7 in the journal Burns compared petrolatum gel to an SSD dressing, with no significant differences in the sample 6.2 ± (SD 2.8) GC: 7.8 ± (SD 2.1); (p = 0.05). A limitation to this study, which the authors acknowledged as a possible cause of bias, was the small patient sample (n = 50), which may have made it difficult to observe the main outcome, since the sample was not blinded during the intervention and only included healthier patients (assuming that this type of intervention can have a different effect when it includes a more heterogeneous population).7 Another study in Iran by Nasiri et al.10 tested an extract of Arnebia euchroma versus SSD, with blinded randomization of two groups of patients (n = 49) and resulting in values of 13.97 ± 5.3 vs. 17.5 ± 6.9 days. Mean age of patients was 39.97 ± 15.6 years, and the majority were women. For patient satisfaction related to treatment site, treatment with Arnebia resulted in 7.2 ± 1.8 days, compared to SSD with 5.3 ± 1.7 (p = 0.001).10 Muang-man et al.11 also found results in favor of the experimental group comparing SSD to Aquacel® Hidrofiber, a carboxymethylcellulose dressing that promotes debridement and antimicrobial protection against vancomycin-resistant Enterococci (VRE) and methicillin-resistant Staphylococcus aureus (MRSA) for up to 14 days.11 Patients with up to 15% of burned body surface area were randomized (n = 70) from December 2006 to February 2008. The authors found shorter healing time in the experimental group versus controls (10 ± 3 vs. 13.7 ± 4.3 (p <0.02).11 In 2007, Homann et al.13 performed a prospective study at Bergmannsheil University Hospital in Germany, comparing a liposome hydrogel with polyvinylpyrrolidone iodide versus SSD. Forty-seven patients were randomized, all of whom were white, with mean age 37.2 ± 17.7 years. Complete healing time was shorter in the experimental group 9.9 (SD 4.5) days, compared to 11.3 (SD 4.9) in the control group. However, in relation to study safety, there were 20 adverse effects related to treatment, 14 of which local, with six systemic adverse events, all related to laboratory results such as gamma glutamyl transferase, leukocytes, hemoglobin, thyroxine, and thyroid stimulating hormone, but not clinically relevant and that could not be related directly to the experimental treatment.13 In 2007, Huang et al.11 used Acticoat™, a silver nanocrystal mesh that enhances healing and protects against more than 150 pathogens, including Pseudomonas, MRSA, and VRE. This was a multicenter study with (n = 98) randomized patients followed for 20 days, with a mean age of 36.81 ± 2.49 years. Healing time was shorter in the group treated with Acticoat, with 12.42 ± (SD 5.40) days versus 15.79 ± (SD 5.60) in the SSD group. The Acticoat dressing was considered highly promising because it can be kept on the wound for up to 3 days, so that patients do not need to bother with changing the dressing daily, thereby reducing wound stress and local pain.14 Medical advances in recent years have led to a major reduction in the number of burn wounds, thus allowing significant overall improvement in patient care.18 Prior to the use of topical antimicrobials, the overall mortality rate in burn victims ranged from 38% to 45%, reduced to 14% to 25% since the introduction of these drugs.18 Sulfadiazine has shown excellent bactericidal activity with its commercially available cream formulation and is well accepted and used by numerous burn centers.19
This review may have had some limitations due to the fact that the cited studies had some characteristics and biases that may have altered the results, such as small patient samples, incorrectly blinded studies, and heterogeneous groups, which may have compromised the detection of significant differences between the tested interventions. Regarding possible study biases, there were five studies in patients with first-degree superficial burns and six studies in patients with second-degree superficial burns, both with at least 10% burned body surface. It is estimated that in studies including more severe cases, healing would take longer when compared to less severe cases and would involve less local pain and fever and lower risk of infection. Since there are different products with silver sulfadiazine available on the market (and used in many hospitals and burn centers throughout the country), the observed effects can vary. Several commercial products have been compared to silver sulfadiazine in clinical trials, including Aloe vera, Centiderm, Aquacel, and others. However, these products were not observed alone in this study, but jointly, in a way that demonstrated efficacy compared to SSD. Therefore, it may be too early to determine their actual single effectiveness, which would require further studies of each specific product. In order to minimize errors and increase accuracy, new clinical trials should be performed with more significant and homogeneous samples to compare groups. Nevertheless, this review was able to provide new perspectives for more effective burn treatments, with reductions in pain, inflammation, and length of hospital stay.
ConclusionsThis systematic review and meta-analysis provide evidence of the efficacy of other burn treatments available on the market compared to silver sulfadiazine. Although sulfadiazine treatment has been used in burn patients for years because of its antimicrobial properties, it can be switched or used in combination with other forms of treatment described in this study in order to improve patients’ quality of life and decrease hospital costs. The authors thus hope that this research will help professionals in clinical practice and government agencies to develop strategies and investments in order to improve quality of life of burn patients, proposing new effective treatments and thereby decreasing sequelae and deaths from burns.