Myeloid leukemia cutis is the terminology used for cutaneous manifestations of myeloid leukemia.
ObjectiveThe purpose of this study was to study the clinical, histopathological and immunohistochemical features of myeloid leukemia cutis.
MethodsThis was a retrospective study of clinical and pathological features of 10 patients with myeloid leukemia cutis.
ResultsOne patient developed skin lesions before the onset of leukemia, seven patients developed skin infiltration within 4-72 months after the onset of leukemia, and two patients developed skin lesions and systemic leukemia simultaneously. Of these patients, five presented with generalized papules or nodules, and five with localized masses. The biopsy of skin lesions showed a large number of tumor cells within the dermis and subcutaneous fat layer. Immunohistochemical analysis showed strong reactivity to myeloperoxidase (MPO), CD15, CD43 and CD45 (LCA) in most cases. NPM1 (nucleophosmin I) and FLT3-ITD (Fms-like tyrosine kinase 3-internal tandem duplication) mutations were identified in one case. Five patients with acute myelogenous leukemia and one patient with chronic myelomonocytic leukemia died within two months to one year after the onset of skin lesions.
Study limitationsThis was a retrospective and small sample study.
ConclusionsIn patients with myelogenous leukemia, skin infiltration usually occurs after, but occasionally before, the appearance of hemogram and myelogram abnormalities, and the presence of skin infiltration is often associated with a poor prognosis and short survival time. myeloid leukemia cutis often presents as generalized or localized nodules or masses with characteristic pathological and histochemical findings.
Leukemia cutis (LC) is the term used for cutaneous manifestations of leukemia.1 The clinical and morphological findings have a wide range of cutaneous manifestations and may present with nodular lesions and plaques. Rare manifestations include erythematous macules, blisters and ulcers which can each occur alone or in combination.2 The lesions may be localized or disseminated and can occur on any site of the skin.2 Histologic features of LC vary with the type of leukemia. The infiltrate of neoplastic leukocytes may be perivascular, nodular and diffuse, or densely surrounding the eccrine glands. The diagnosis of LC is verified by skin biopsy and immunophenotyping. The purpose of this study was to study the clinical, histopathological and immunohistochemical features of 10 patients with myeloid leukemia cutis (MLC).
MethodsCase selectionThis was a retrospective study of clinical, histopathological and immunohistochemical features of 10 patients with MLC. From 1316 patients with ML searched from the database of Peking Union Medical College Hospital from July 2002 to April 2015, 10 patients with MLC were eligible. Morphological, histopathological, immunohistochemical and cytogenetic analyses were performed by the specialists from the Departments of Hematology, Dermatology, and Pathology. Based on these criteria, 10 cases were selected for the study.
Clinical dataClinical data include age, sex, interval between skin lesion and systemic leukemia, clinical appearance and distribution. Myeloid leukemia cases were classified according to the French-American-British (FAB) classification in the Department of Hematology.
Histologic and immunohistochemical dataFor each case, paraffin blocks were available with H&E and Giemsa stains. All cases were reviewed by the pathologists in the Department of Dermatology and Pathology. Sixteen antibodies were tested: myeloperoxidase (MPO), CD13, CD43, CD45 (LCA), CD45RO, CD68, CD15, CD3, CD4, CD30, CD33, CD34, CD99, CD117, AE1/AE3 (epithelium) and vimentin. Most of the atypical lymphocytes stained positively with MPO and CD markers such as CD3, CD4, CD13, CD15, CD33 and CD117, thereby confirming the presence of leukemia cells.
ResultsGeneral dataThe ten cases included one patient with AML-M1 (acute myelocytic leukemia), one patient with AML-M2, three patients with AML-M3, one patient with AML-M4, one patient with chronic myelomonocytic leukemia (CMML) and three patients with granulocytic sarcoma (GS). The male-female ratio was 3:2. Mean age was 49.3 years at the time of diagnosis. One patient developed skin lesions 8 months earlier than the onset of leukemia, seven patients developed skin infiltration within 4-72 months after the onset of leukemia, and two patients developed skin lesions and systemic leukemia simultaneously (Table 1).
Clinical appearance of the patients with myeloid leukemia cutis
| Patient No. | Age(y)/ Sex | Type/subtypes | Duration before diagnosis (mo) | Clinical appearance and distribution |
|---|---|---|---|---|
| 1 | 59/F | AML-M1 | 12 | Multiple papules on trunk, arms and legs (figure 1a) |
| 2 | 28/M | AML-M2 | -8 | Crusts and ulcerated tumor on left thigh, nodule on right thigh, multiple papules on groin (figure 1b) |
| 3 | 67/M | AML-M3 | 4 | Multiple papules, nodules on trunk and extremity |
| 4 | 26/F | AML-M3 | 18 | Nodules on face, trunk, limbs, and mouth |
| 5 | 42/M | AML-M3 | 20 | Bleeding tumor on bilateral external auditory canal and right temple |
| 6 | 81/M | AML-M4 | 0 | Nodules on trunk, limbs, and scrotum (figure 1c) |
| 7 | 68/M | CMML | 20 | Multiple papules on trunk and extremity (figure 1d) |
| 8 | 39/M | GS | 72 | Bleeding gingival mass on right maxillary |
| 9 | 32/F | GS | 0 | Ulcerated tumor on head |
| 10 | 51/F | GS | 56 | Gengival mass on left maxillary |
AML, acute myelocytic leukemia; CMML, chronic myelomonocytic leukemia; GS, granulocytic sarcoma; No., number.
Cutaneous manifestations of leukemia vary in a broad spectrum. Nodules (40.0%, 4/10), papules (33.3%, 3/10), and masses (40.0%, 4/10) can most commonly be seen in 10 patients (Figure 1). Macules (10.0%, 1/10) and ulcers (0/10) were uncommon lesions in MLC. Nodules (66.7%, 4/6) can most commonly be seen in AML, and masses (100%, 3/3) were the most common lesions in GS. The face (40.0%, 4/10), extremities (40.0%, 4/10) and trunk (50.0%, 5/10) were the most common locations of MLC. In addition, AML and CMML lesions are often located on extremities and trunk (85.7%, 6/7). Most lesions (60.0%, 6/10) were disseminated, while four cases (40%, 4/10) were localized. Localized lesions were the most common lesions in GS (100%, 3/3), followed by AML (14.3%, 1/7) (Table 1).
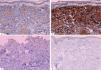
Histopathological, immunohistochemical and molecular featuresHistopathology examination of the lesion was performed on all patients. The biopsy of skin lesions showed a large number of diffuse tumor cells within the dermis and subcutaneous fat layer (Figure 2). Immunohistochemical analysis showed strong reactivity for MPO, CD15, CD43 and CD45 (LCA) in most cases, and variable staining for CD34, CD45RO, CD68 and CD99. However CD4, CD30, CD117, AE1/AE3 (epithelium) and vimentin were only positive in case 10 with GS. MPO(60%, 6/1), CD15 (50%, 5/10) and CD43 (50%, 5/10) were the most common positive stains in both AML and CML. Additionally, two GS patients were positive for MPO, CD68 and Bcl-2 (Figure 3). Overall results of immunohistochemical analysis are given in table 2. In this study, mutational analysis was performed on all cases on the FLT3-ITD (Fms-like tyrosine kinase 3-internal tandem duplication) and NPM1 (Nucleophosmin1) coding regions. Both NPM1 and FLT3-ITD mutations were only positive in case 1, but negative in other patients. (Table 3)
A - Diffuse dermal atypical myeloid infiltrates involving subcutis with Grenz zone. (Hematoxylin & eosin, x10); B - Infiltrates were composed of small to medium sized mononuclear cells with atypical cytological features, including nuclear hyperchromasia, coarse chromatin pattern, and thickened nuclear membrane. (Hematoxylin & eosin, X40)
Immunohistochemical features of skin lesions in 10 patients with myeloid leukemia cutis
| Stain/Patient No | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| Type/subtype | AML | AML | AML | AML | AML | AML | CMML | GS | GS | GS |
| M1 | M2 | M3 | M4 | M5 | M6 | |||||
| MPO | + | + | + | + | + | + | + | |||
| CD3 | - | - | - | |||||||
| CD4 | + | |||||||||
| CD13 | + | + | ||||||||
| CD15 | + | + | + | + | ||||||
| CD30(Ki-1) | - | + | ||||||||
| CD33 | + | |||||||||
| CD34(Vessel) | + | + | + | |||||||
| CD43 | + | + | + | + | ||||||
| CD45(LCA) | + | + | + | + | + | |||||
| CD45RO | + | - | ||||||||
| CD79a | + | |||||||||
| CD68 | + | + | + | + | ||||||
| CD99 | + | |||||||||
| CD117 | + | |||||||||
| AE1/AE3 (epithelium) | - | + | ||||||||
| Vimentin | + | |||||||||
| Bcl-2 | + | + |
AML, acute myelocytic leukemia; CMML, chronic myelomonocytic leukemia; GS, granulocytic sarcoma; MPO, Myeloperoxidase.
Molecular features and prognosis of 10 patients with myeloid leukemia cutis
| Case | Age(y)/Sex | NPM1 | FLT3-ITD/FLT3-TKD | Prognosis |
| 1 | 59/F | Type A mutation | Insertion mutation | Alive |
| 2 | 28/M | - | - | Dead |
| 3 | 67/M | - | - | Dead |
| 4 | 26/F | - | - | Dead |
| 5 | 42/M | - | - | Dead |
| 6 | 81/M | - | - | Dead |
| 7 | 68/M | - | - | Dead |
| 8 | 39/M | - | - | Lost follow-up |
| 9 | 32/F | - | - | Lost follow-up |
| 10 | 51/F | - | - | Alive |
FLT3-ITD, Fms-like tyrosine kinase 3-internal tandem duplication; NPM1, Nucleophosmin 1.
Since skin lesions are generally indicative of a poor prognosis, we then tried to correlate the occurrence of cutaneous leukemia lesions with clinical prognosis. In this study, six of 10 patients (60.0%, 6/10) died after the onset of skin lesions within two months to one year. Among these six patients, included five patients with AML and one patient with CMML. The average interval between the onset of MLC and death was 4.75±3.458 months (Table 3).
DiscussionMLC often presents as generalized or localized nodules or masses with characteristic pathological and histochemical manifestations. Particularly, the presence of skin infiltration is often associated with a poor prognosis and short survival time.
Skin manifestationsAmong the 10 patients in this study, five presented with generalized erythematous infiltrated papules, maculopapules or nodules, and five presented with localized masses. Kaddu et al3 reported that MLC presented as solitary or multiple reddish to violaceous papules, plaques, and nodules (17 lesions), or as a generalized erythematous maculopapular eruption (9 lesions). In this study, nodules, papules and masses are commonly seen in 10 patients. Nodules are most frequently seen in AML, whereas papules were the most common lesion in CML. In Kang’s study, the most commonly clinically observed skin lesions were nodules, papules, and plaques, in decreasing order.4 Specifically, case 8 and 10 in our study only presented with oral lesions, which occurred after the onset of leukemia. Oral manifestations occur frequently in leukemia patients and may present as initial evidence of the disease or its relapse. These oral lesions may either be the result of direct infiltration of leukemia cells (primary) or secondary to underlying thrombocytopenia, neutropenia, or impaired granulocyte function. The distribution of skin lesions was similar in most kinds of leukemia. In this study, the face, extremities and trunk were the most frequent locations of MLC. AML and CMML lesions were often located on extremities and trunk, while GS lesions did not. Most lesions were disseminated, while four cases were localized. Watson et al.5 found that the distribution of skin lesions was found to be similar in patients with AML, CML and CLL (chronic lymphocytic leukemia), there was no apparent predilection for the sites of involvement. In a study of 75 patients with LC, they also described similar distribution features.4 In this study, one patient developed skin lesions before the onset of leukemia, seven patients developed skin infiltration within 4-72 months after the onset of leukemia, two patients developed skin lesions and systemic leukemia simultaneously. In patients with myelogenous leukemia, skin infiltration usually occurs after, but occasionally before, the appearance of abnormal haemogram and myelogram.5
Histopathological, immunohistochemical and molecular dataThe histopathological diagnosis of LC is based on an evaluation of the pattern of distribution, cytologic findings, and immunohistochemical characteristics of the tumor cells.2 In Klco’s6 study, one-third of patients with LC had a diffuse pattern of infiltration with involvement of the superficial and deep dermis. In this study, the biopsy of skin lesions showed a large number of tumor cells within dermis and subcutaneous fat layer. In addition, after two experienced pathologists assessed HE stain sections alone, case 8, 9, and 10 can be diagnosed as GS. Immunohistochemical analysis showed strong reactivity for MPO, CD15, CD43 and CD45 (LCA) in most cases, and variable staining for CD34, CD45RO, CD68 and CD99. However CD4, CD30, CD117, AE1/AE3 (epithelium) and vimentin were only positive in case 10. Myeloid disorders can be diagnosed when there is an absence of specific B-cell and T-cell markers and there is positive staining with markers of myelomonocytic lineage such as CD34, CD15, CD68, myeloperoxidase and CD117. MPO which is a sensitive marker of myeloid lineage3, showed strong reactivity in case 1, 2, 3, 5, 7, 8 and 9. Myeloid antigen (such as CD13, CD15, CD33, CD34 and CD117) were partially positive in case 4, 6 and 10, confirming the myeloid lineage of the cells. In addition, partial monocyte/macrophage markers (CD45RO, CD68) were positive in case 1, 2, 6, 9 and 10. It’s suggested that simultaneous expression of lysozyme, MPO, CD45, CD43, and CD74 militates in favor of a diagnosis of specific cutaneous infiltrate of myelogenous leucemia.3 In this study, case 1 (AML-M1) and 7 (CMML) had simultaneous expression of MPO, CD43 and CD45, while case 2 (AML-M2), 3 (AML-M3), 5 (AML-M3) and 7 (CMML) had simultaneous expression of MPO and CD43. Therefore, immunohistochemistry is valuable to diagnose different kinds of skin lesions in patients with AML and CML. But it is not possible to classify the different forms of leukemia by skin biopsy alone; additional cytochemical and molecular genetic studies (e.g., bone marrow biopsy) are needed.7 The gene mutation of FLT3 and NPM1 in patients with acute myeloid leukemia is common. These two mutations are frequently associated. Approximately 40% of patients with NPM1 mutations also carry FLT3-ITD, NPM1 mutations may have a good prognostic impact, while FLT3-ITD mutations may have a bad prognostic impact.8,9 At present, information about these mutations are assuming as much significance as cytogenetics in decisions about therapy.10 In this study, positive FLT3-ITD and NPM1 were only seen in case 1. Therefore, it is difficult for us to analyze the correlation between the gene results and the prognosis of the patients.
PrognosisSkin lesion is generally indicative of a poor prognosis, and is associated with transformation into a blastic phase and suggests disease progression.7 Kaddu et al.3 reported that all patients with CML and AML with adequate follow-up died within 24 months after the onset of skin lesions (mean survival, AML: 7.6 months; CML: 9.4 months). Kang et al.11 reported that the prognosis of LC was poor within one year. In Watson’s study, the time from the appearance of LC to death ranged from three months (CML) to 39 months (CLL). In this study, six patients with AML and one patient with CMML died within two months to one year after the onset of skin lesions.5 The average interval between the onset of MLC and death was 5.6 months, and the result in Kang’s study was 8.3 months.4
ConclusionThe presence of skin infiltration is often associated with a poor prognosis and short survival time. At present, the diagnosis of LC is often made in retrospect once leukemia becomes present clinically.12 Therefore, it is imperative that clinicians be familiar with features of cutaneous lesions that may indicate an underlying malignancy.13 A skin biopsy can be the first indication of the presence of leukemia cutis in the patients with systemic leukemia.14,15 This was a retrospective and small sample study, which is its limitation.
AcknowledgmentThe authors would like to acknowledge the valuable comments and suggestions from Dr. Tao Wang.
Financial support: This study was supported by China National Natural Science Funds (No. 81371731) and the Education Reform Projects of Peking Union
Medical College (No. 2016zlgc0106).
Conflict of interest: None.