There are scarce studies in the literature about hyaluronic acid in systemic autoimmune myopathies.
ObjectivesTo analyze the serum level of hyaluronic acid in patients with dermatomyositis and polymyositis.
MethodsCross-sectional study, single-center, that evaluated hyaluronic acid in 18 dermatomyositis and 15 polymyositis (Bohan and Peter criteria), newly diagnosed, with clinical and laboratory activity, with no previous drug treatment. The patients were also age-, gender- and ethnicity-matched to 36 healthy individuals. The hyaluronic acid was analyzed by ELISA/EIA kit anti-hyaluronic acid.
ResultsThere was a higher serum level of hyaluronic acid in patients with autoimmune myopathies, in relation to control group (P<0.05). Moreover, the serum level of this glycosaminoglycan was higher in dermatomyositis, when compared to polymyositis. Both groups were comparable with regard to demographic, clinical and laboratory data, except for the presence of skin lesions in the first group.
Study limitationsThe presence of hyaluronic acid in cutaneous lesions, particularly of patients with dermatomyositis, was not analyzed neither quantified. In addition, due to disease rarity and the establishment of strict inclusion and exclusion criteria, there was a small sample in the present study.
ConclusionsAs an example of others systemic autoimmune diseases, it is possible that the hyaluronic acid is involved in the pathogenesis of autoimmune myopathies, and particularly when associated with cutaneous lesions.
Dermatomyositis (DM) and polymyositis (PM) are idiopathic inflammatory myopathies, characterized by progressive symmetrical and predominantly proximal muscle weakness. Additionally, DM has multiple cutaneous presentations, such as heliotrope, Gottron’s papules, periungual hyperemia, “mechanic hands”, ulcers, vasculitis, V-neck sign, shawl sign, calcinosis, Raynaud phenomenon, among others.1-4
Hyaluronic acid (HA) is one of the main elements of the extracellular matrix, with a substantive participation in the synovium, such as lubrication of the joints, hydration and supply of a matrix that allows cellular migration.5 HA does not only provide a structure that allows the internal growth of fibroblasts and blood vessels, but also regulates multiple aspects of cellular mechanisms related to tissue repair, such as the activation of inflammatory cells to induce the immunological response.5
Different studies have observed the rise in serum levels of HA in many systemic autoimmune conditions, including systemic sclerosis, psoriatic arthritis and systemic lupus ertythematosus.6-9 However, the study of HA in DM and PM is scarce.9-13
The first descriptions related to the rise in HA serum levels in patients with DM were by Kubo et al10,11 However, in one of the studies, these authors only followed two patients, both Asians, and one of them had malignancy-associated DM; in addition, these patients had a decrease in disease activity and of HA levels after the use of glucocorticoids.11
In another study, HA was analyzed in conjunction with many systemic autoimmune conditions, with DM among them. However, DM patients had association with malignancies and pulmonary fibrosis, what could have contributed to the rise in HA levels.10 Besides, these authors did not mention the use of glucocorticoids or immunosuppressants while analyzing serum HA.
Recently, two studies also showed a rise in the serum level of HA in patients with DM and PM.12,13 Nonetheless, as a limitation, these studies included patients under treatment with drugs and/or stable, what could have interfered in the actual reading of HA serum levels.
Therefore, in the present study, the serum level of HA was evaluated specifically in patients recently diagnosed with DM and PM, with clinical and laboratory activity and with no previous drug treatment.
MethodsThis study was based in a single-center cross-sectional design, where the level of HA was assessed in consecutive 18 DM cases and 15 PM cases, from 2010 to 2013. All patients were initially admitted for investigation of progressive, symmetrical, predominantly proximal muscle weakness with no apparent cause, associated to a rise in the serum levels of the muscle enzymes creatine phosphokinase (CPK) and aldolase. All patients had purely myopathic pattern on the electroneuromyography and muscle biopsy consistent with inflammatory myopathy, characterizing the picture of idiopathic inflammatory myopathy - Bohan and Peter criteria.3 Besides, 18 patients (DM) had classical cutaneous lesions of heliotrope and/or Gottron’s papules.
Patients had less than 1 year between the diagnosis and the onset of disease, defined in this study as recently diagnosed, and had no previous drug treatment specific for muscle disease.
During the period of clinical and laboratory investigation, malignancies and/or infectious causes were excluded. Moreover, as the internal protocol of the Service, blood samples were collected from the patients and immediately centrifuged at 4°C, 3000rpm for 15 minutes and stored in a freezer at - 80°C.
Thirty-six healthy adult voluntaries were included as the control group, matched by sex, age and ethnicity, for the same period of the study.
The study was approved by the local Ethics Committee (number 22401).
The following patient data were systematically revised from electronic files with pre-configured and standardized parameters:
a) Demographic data: current age, ethnicity and sex;
b) Clinical features: time between the diagnosis of the disease and the onset of symptoms; constitutional symptoms; joint (arthralgia or arthritis), cardiac or pulmonary (progressive dyspnea associated to the presence of changes in the pulmonary parenchyma, seen on computed tomography: interstitial pneumopathy, ground-glass opacities or honeycomb lung) involvement; limbs muscle strength (grade 0: absence of muscle contraction; grade I: signs of mild contraction; grade II: normal range movements, but not overcoming gravity; grade III: normal range movements against gravity; grade IV: complete movement against gravity and some degree of resistance; grade V: complete movement against resistance and against gravity); cutaneous (heliotrope, Gottron’s papules, vasculitis, facial rash, V-neck sign, shawl sign, calcinosis, periungual hyperemia, Raynaud phenomenon);14
c) laboratory data: HA serum level was determined in blood samples previously stored, using a specific anti-HA ELISA kit, according to the manufacturer’s protocol; serum level of CPK (reference values: 24 to 173U/L) and aldolase (1.0 to 7.5U/L), using automated kinetics.
Statistical analysisDemographic data and clinical characteristics were expressed by mean ± standard deviation for continuous variables or as frequency (%) for categorical variables. Median (percentile 25% - 75%) was calculated for continuous variables with non-normal distribution. Kolmogorov-Smirnov test was used to evaluate the normal distribution of each continuous parameter analyzed. The comparison of the categorical parameters between the groups was performed using the chi-square test or Fisher’s exact test. For continuous variables, Mann-Whitney or t-Student tests were used. For the correlation between the serum level of HA and the continuous parameters, the correlation of Spearman was used. P values of <0.05 were considered significant. All analyses were performed with SPSS 15.0 statistical software (Chicago, EUA).
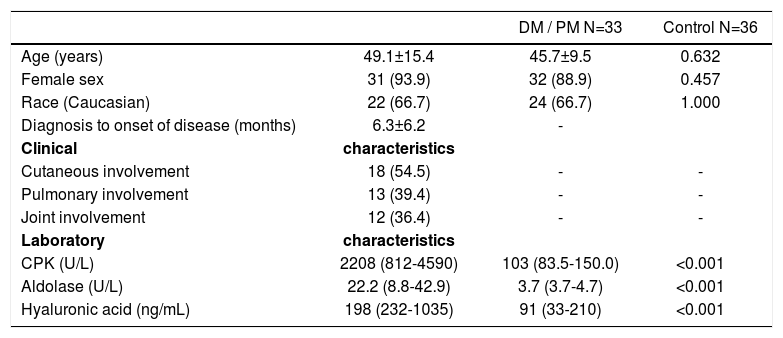
ResultsTable 1 presents demographic, clinical and laboratory data for the patients with DM and PM and data of the control group. Age, gender and ethnicity distribution was comparable between both groups (P>0.05). Mean time between the diagnosis of the condition and onset of symptoms was of 6.3 months. Constitutional symptoms were present in about 3/4 of patients, followed by cutaneous, pulmonary and joint manifestations. The levels of muscle enzymes were higher in patients with DM and PM when compared to the control group (P<0.001).
Demographic, clinical and laboratory characteristics of the patients with dermatomyositis and polymyositis compared to healthy individuals (control)
| DM / PM N=33 | Control N=36 | ||
|---|---|---|---|
| Age (years) | 49.1±15.4 | 45.7±9.5 | 0.632 |
| Female sex | 31 (93.9) | 32 (88.9) | 0.457 |
| Race (Caucasian) | 22 (66.7) | 24 (66.7) | 1.000 |
| Diagnosis to onset of disease (months) | 6.3±6.2 | - | |
| Clinical | characteristics | ||
| Cutaneous involvement | 18 (54.5) | - | - |
| Pulmonary involvement | 13 (39.4) | - | - |
| Joint involvement | 12 (36.4) | - | - |
| Laboratory | characteristics | ||
| CPK (U/L) | 2208 (812-4590) | 103 (83.5-150.0) | <0.001 |
| Aldolase (U/L) | 22.2 (8.8-42.9) | 3.7 (3.7-4.7) | <0.001 |
| Hyaluronic acid (ng/mL) | 198 (232-1035) | 91 (33-210) | <0.001 |
CPK: creatine phosphokinase; DM: dermatomyositis; PM: polymyositis. Results are expressed as mean ± standard deviation, median (interquartile 25%-75%) or percentage (%).
HA serum levels were significantly raised in patients with DM and PM when compared to the control group: 198 (232-1035) versus 91 (33-210) ng/mL; P<0.001.
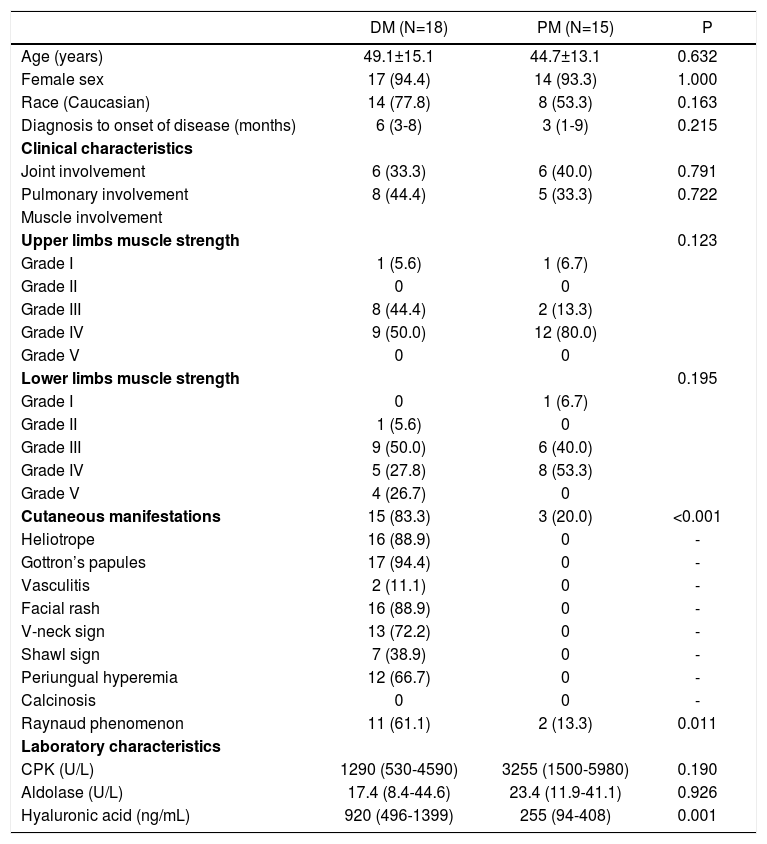
As an additional analysis, demographic and clinical data of patients with DM and PM were compared to each other. The data were similar between the groups, except for the presence of cutaneous lesions, that occurred only in DM cases. Besides, HA levels were significantly elevated in patients with DM when compared to the PM group: 920 (496-1399) versus 255 (94-408)ng/mL; P=0.001.
When analyzing each condition (DM or PM), serum level of HA was not correlated to any of the parameters shown in table 2.
Demographic, clinical and laboratory characteristics of patients with dermatomyositis and polymyositis
| DM (N=18) | PM (N=15) | P | |
|---|---|---|---|
| Age (years) | 49.1±15.1 | 44.7±13.1 | 0.632 |
| Female sex | 17 (94.4) | 14 (93.3) | 1.000 |
| Race (Caucasian) | 14 (77.8) | 8 (53.3) | 0.163 |
| Diagnosis to onset of disease (months) | 6 (3-8) | 3 (1-9) | 0.215 |
| Clinical characteristics | |||
| Joint involvement | 6 (33.3) | 6 (40.0) | 0.791 |
| Pulmonary involvement | 8 (44.4) | 5 (33.3) | 0.722 |
| Muscle involvement | |||
| Upper limbs muscle strength | 0.123 | ||
| Grade I | 1 (5.6) | 1 (6.7) | |
| Grade II | 0 | 0 | |
| Grade III | 8 (44.4) | 2 (13.3) | |
| Grade IV | 9 (50.0) | 12 (80.0) | |
| Grade V | 0 | 0 | |
| Lower limbs muscle strength | 0.195 | ||
| Grade I | 0 | 1 (6.7) | |
| Grade II | 1 (5.6) | 0 | |
| Grade III | 9 (50.0) | 6 (40.0) | |
| Grade IV | 5 (27.8) | 8 (53.3) | |
| Grade V | 4 (26.7) | 0 | |
| Cutaneous manifestations | 15 (83.3) | 3 (20.0) | <0.001 |
| Heliotrope | 16 (88.9) | 0 | - |
| Gottron’s papules | 17 (94.4) | 0 | - |
| Vasculitis | 2 (11.1) | 0 | - |
| Facial rash | 16 (88.9) | 0 | - |
| V-neck sign | 13 (72.2) | 0 | - |
| Shawl sign | 7 (38.9) | 0 | - |
| Periungual hyperemia | 12 (66.7) | 0 | - |
| Calcinosis | 0 | 0 | - |
| Raynaud phenomenon | 11 (61.1) | 2 (13.3) | 0.011 |
| Laboratory characteristics | |||
| CPK (U/L) | 1290 (530-4590) | 3255 (1500-5980) | 0.190 |
| Aldolase (U/L) | 17.4 (8.4-44.6) | 23.4 (11.9-41.1) | 0.926 |
| Hyaluronic acid (ng/mL) | 920 (496-1399) | 255 (94-408) | 0.001 |
CPK: creatine phosphokinase; DM: dermatomyositis; PM: polymyositis. Results are expressed as mean ± standard deviation, median [interquartile 25%-75%] or percentage (%).
In the present study, a high level of HA was observed in patients with DM and PM in comparison to healthy individuals. Its level was higher in DM patients, who had cutaneous involvement as opposed to PM patients.
Even though there are scarce studies in the literature, the presence of an elevated level of HA in autoimmune myopathy patients has already been demosntrated.10-13 However, as a differential, the present study analyzed this glycosaminoglycan specifically in patients with clinical and laboratory activity without previous drug treatment. Moreover, only recently diagnosed cases were included, as well as a control group matched by sex, age and ethnicity.
Victorino et al.12 observed a strong association between HA and cutaneous lesions found in DM (photosensitivity, V-neck sign, shawl sign, periungual telangiectasia). Silva et al.13 noted a positive correlation between the serum level of HA and the activity of patients with PM. However, these authors evaluated patients with and without disease activity, and only under different therapeutic regimens with glucocorticoids and/or immunosuppressants.12,13 In the present study, an association between HA and the different disease parameters was not observed, probably due to the fact that only patients with disease activity (DM or PM) were included. In contrast, because the level of HA was higher in DM than in PM, it is possible to deduct that this glycosaminoglycan is involved not only in the physiopathogenesis of both conditions, but also in the cutaneous lesions of DM.
Many studies have observed the rise in serum levels of HA in other systemic autoimmune conditions such as systemic sclerosis, systemic lupus erythematosus and psoriatic arthritis.6-9 In the latter, HA, besides being present in increased serum levels, correlated to the cutaneous changes, but not to joint involvement.5 Chang et al.9 observed HA accumulation, as well as chondroitin sulfate, specifically in cutaneous lesions from patients with systemic lupus erythematosus and also DM.
HA is a component of the connective tissue matrix and can also be found in the intercellular space and papillary dermis.12,13 It has regulatory and structural roles in the connective tissue, attenuates drainage of synovial fluid, lubricates and absorbs mechanical impacts, and also plays a part in immunological functions.5 In this setting, HA stimulates the release of inflammatory factors such as TNF-α and IL-1β, and fibroblast cytokines, aiding in the inflammatory response and tissue repair.5,15,16
Thus the presented data suggest that HA can also be involved in the processes of systemic immunological/inflammatory responses of patients with DM and PM. Moreover, it can have an important role in the physiopathogenesis of cutaneous lesions of patients with DM, as what happens with psoriatic arthritis patients, promoting tissue repair through inflammatory cells, proliferation of fibroblasts and neoangiogenesis.5,7,8
As a study limitation, the presence of HA was not analyzed nor quantified in cutaneous lesions, particularly in DM patients and, due to the rarity of the condition and strict inclusion and exclusion criteria, there was a small sample in this study.
ConclusionIncreased serum levels of HA are found in patients with DM and PM when compared to healthy individuals. These findings can be associated to the pathophysiological process of these conditions. Most importantly, HA serum levels are increased in DM when compared to PM patients, possibly associated to cutaneous lesions. However, further prospective studies are needed for the better understanding of its true physiopathogenic mechanism.