Erythema ab igne is a reticulated, hyperpigmented dermatosis that arises following chronic infrared exposure. It has been reported with prolonged exposure to heating pads or blankets, hot water bottles, heated furniture, laptop computers, prolonged bathing in hot water, open fires, and wood-burning stoves, among others.1-3 It is usually asymptomatic and resolves with discontinuation of the offending heat source. There are several reported cases of neo-plastic transformation occurring at the affected site.4,5
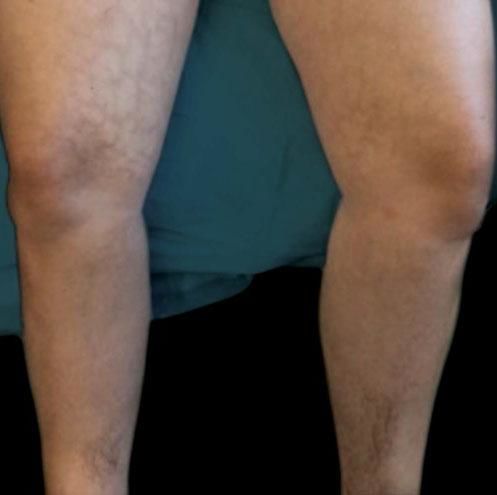
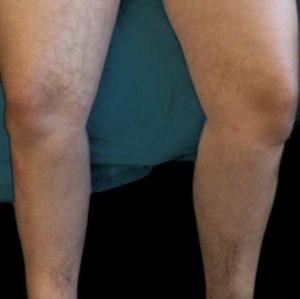
A thirty-four-year-old Caucasian female presented to the dermatology clinic for routine follow-up. The patient had a history of alopecia totalis for the previous four years, which had been treated with a Janus kinase (JAK) inhibitor for the previous five months. The patient experienced regrowth of the majority of her scalp hair, eyebrows, and patches of leg hair. The patient's review of symptoms was essentially negative with the exception of a new rash on her bilateral medial thighs. Given her successful regrowth of hair, the patient was apprehensive that her JAK inhibitor would be discontinued given her developing skin rash. Physical examination of the bilateral medial thighs revealed reticulated, hyperpigmented-to-violaceous patches (Figure 1). The skin texture was normal, and there were no signs of venous stasis affecting the distal lower extremities. The patient was employed in computer science and worked primarily from home. She denied use of a laptop computer being placed on her lap or other common heat sources. However, she did report that for the previous two years she had been using a Japanese kotatsu table as a workspace. The patient's legs rested just under the electric heating element for a considerable amount of time throughout the day. The patient was diagnosed with erythema ab igne, and she was counseled to avoid chronic exposure to heat on her lower extremities.
Erythema ab igne initially begins as transient, blanchable, macular erythema confined to the affected geographic area that reflects that shape and size of the heat source.4 With continued heat exposure, the area then develops into a fixed, reticulated pattern of hyperpigmentation, which can progress to skin atrophy, hyperkeratosis, and sometimes telangiectasias.3 Biopsy of the affected site may reveal a wide array of findings histologically, including nonspecific thinning of the epidermis, blunting of the rete ridges, and altered dermal elastic fibers with dermal hemosiderin and melanin incontinence.3,4 Since these findings are nonspecific, the diagnosis of EAI is usually made clinically as in our case, when reticulated hyper-pigmentation and erythema are present in the context of skin that is chronically exposed to heat.
While EAI is more common in sites of chronic pain, such as the low back or abdomen, there are numerous case reports of EAI arising in other locations following long-term heat exposure. To date, most cases of EAI have described similar lesions caused by heating pads, hot water bottles, laptop computers, and heated massage chairs. While these cases have offered diverse presentations, our case demonstrates another novel presentation of erythema ab igne. With the gaining popularity of kotatsu tables, clinicians should be aware of this possible adverse effect associated with them.
Early identification and education for patients on the risks of using local heat sources chronically, as well as removing the offending heat source when EAI appears are of high priority. Once the heating source is identified, it is important to address the underlying cause for use of the heating agent, whether for pain or warmth. If the reason for use is pain, the underlying cause should be identified and treated. There have been cases of occult gastrointestinal disease and malignancy identified after EAI developed in sites of overlying skin or areas of referred pain.1 Alternatively, if the underlying reason is for warmth, another heating modality should be considered. Body heat instability should also be addressed. In our case, chronic external heat was used in the context of alopecia totalis. These are just a couple examples when clinicians may intervene and offer not only guidance for EAI but also treatment of the underlying cause.
Although there is generally a good prognosis when treating EAI, the risk of malignant transformation by various cells is well documented.4,5 Identifying and avoiding the offending exposure with continued monitoring of the affected area is important. Additionally, if any area affected with EAI demonstrates surface changes or symptoms, then biopsy should be obtained to evaluate for malignant transformation.