Background: A high prevalence of leprosy among children under 15 years of age indicates the need to implement actions to prevent new cases of the disease. Serological tests have been developed with the aim of helping to control the disease by indicating, through seropositivity, the presence of infection.
Objectives: To analyze the prevalence and factors associated with seropositivity rate for anti-NDO-LID antibodies in children under 15 years of age, contacts of leprosy patients.
Methods: We performed a cross-sectional study with 210 children under 15 years old of age. Of them, 50 were household contacts and 160 were neighborhood contacts living in the municipality of Cuiabá, state of Mato Grosso, in 2016. The data were obtained from interviews and the NDO-LID rapid test during home visits from February to July 2016. For the analysis, we used Poisson regression and prevalence ratio.
Results: Seropositivity in contacts was 6.2%. Variables associated with seropositive tests included sex (PR = 1.05; 95% CI: 1.01 -1.08), race/skin color (PR = 0.95; 95% CI: 0.90 - 0.99), residence area (PR = 1.05; 95% CI: 1.01 - 1.09), and number of people per household (PR = 1.06; 95% CI: 1.02 - 1.08).
Study limitations: The small sample size, besides leading to wide confidence intervals, may have been a limitation for the identification of associated factors.
Conclusion: The prevalence of seropositivity was high. Variables associated with NDO-LID seropositivity included female sex, not to be brown skinned, live in urban areas, and live with five or more people.
Leprosy is a chronic and highly disabling transmissible disease caused by infection with Mycobacterium leprae (M. leprae). The disease affects the skin and peripheral nerves.1
Children are considered a high-risk group for infection and disease due to the incomplete development of their immune system, especially those exposed to the bacillus in their home or neighborhood environment.2-4
The disease, when it affects children under 15, can affect growth and development, cause physical limitations and sequelae, and lead to hospitalizations. It can also cause changes in the daily life of patients and their families, affect school life, cause losses in social relationships, jeopardize recreational activities, and even trigger psychological problems.5
In 2015, 18,796 new cases of leprosy were recorded in children under 15 years of age worldwide.6 Brazil, in that same year, had 1,942 new cases in that same age group and reported an annual detection rate of 4.28/100 thousand population between zero to 14 years of age, which is considered high according to parameters of the Brazilian Ministry of Health.1,6,7 The state of Mato Grosso remains hyperendemic, and this pattern of hyperendemicity has been observed in the last ten years in Cuiabá, a region with a high concentration of cases and, consequently, at greater risk for the disease.8
Leprosy control has been a challenge, and several serological tests have been developed with the aim of helping to control the disease, among them, the anti-natural octyl disaccharide-leprosy IDRI diagnostic (NDO-LID).
NDO-LID is a rapid immunochromatographic assay with a simple add-and-read procedure that, by identifying specific antibodies against the bacillus that causes the disease, allows the early detection of M. leprae infection.9,10 Its use in contacts of leprosy patients has been suggested since they are a population at high risk for the disease.9,10
The use of NDO-LID rapid test among the population under 15 years of age is rare and little is known about the factors associated with positive results. Most research with the tests is performed on populations older than 15 years of age and restrict the approach to intrahousehold contacts.9-14
The present study aims to analyze the prevalence and factors associated with seropositivity rates in the anti-NDO-LID rapid test in children under 15 years of age, contacts of leprosy patients.
MethodWe performed a cross-sectional study conducted with children under 15 years of age, contacts of leprosy patients, living in the municipality of Cuiabá, state of Mato Grosso, Brazil, in 2016.
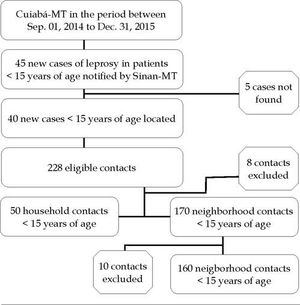
The subjects were selected from the initial identification of 45 cases of leprosy in children under 15 years of age reported in the Brazilian Notifiable Diseases Surveillance System, Mato Grosso division (SINAN-MT) from 2014 to 2015. Of these cases, 5 patients were not found. From the 40 remaining cases (75% treated and 25% under treatment), we were able to identify 228 contacts younger than 15 years. Patients who failed to attend the health unit at a scheduled date after two home visits (n = 8 contacts), and neighborhood contacts who were also household contacts (n = 10) were also excluded, making a total of 210 analyzed children under the age of 15 years (50 household contacts and 160 neighborhood contacts residing within a radius of up to 100 meters from the residence of a leprosy patient under 15 years of age, Figure 1).
The data collection was carried out by nursing and medical professors and students from February to July 2016. We used a structured questionnaire to obtain socioeconomic, demographic, and cohabitation information from leprosy patients. When necessary, the interviews were done with parents and/or legal guardians.
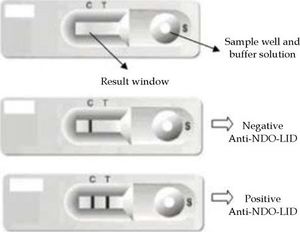
The NDO-LID rapid test was performed from a drop of whole blood loaded into the sample well of the device, followed by the injection of 2-3 drops of buffer solution, with a visual readout of the result within 20 minutes by two independent readers. A single colored line in the results window indicated a negative result. Two colored lines, on the other hand, indicated a positive result. If there were no visible colored lines, the result was considered invalid and the test was repeated (Figure 2).
We conducted independent statistical analyzes for household and neighborhood contacts. The result of the NDO-LID test was considered as a dependent/response variable, which was categorized as positive or negative. Socioeconomic and demographic variables (age, sex, race/skin color, residence area, and housing situation) and co-habitation variables (number of people per household, number of children under 15 years per household, and length of residence) were considered as independent/exposure variables.
Race/skin color was categorized as brown and non-brown (white, black, Asian, and indigenous), and was self-identified during the interview.15 The data were double entered onto EpiInfo version 3.5.2. For the statistical analysis, we used EpiInfo version 7 and Stata version 8.0. Seropositivity prevalence was calculated separately for household contacts, neighborhood contacts, and all contacts together.
In order to identify associations between dependent and independent variables, we used prevalence ratio (PR) with confidence interval set at 95% (95% CI) using the Mantel-Haenszel test or Fisher’s exact test. The variables that had p values smaller than or equal to 0.20 in this bivariate analysis were adjusted in the Poisson multiple regression model, being gradually removed by the backward method. Only the variables that had a statistical significance of 5% (p < 0.05) were included in the final multiple model.
The present study is linked to a matrix project approved by the Research Ethics Committee of the Universidade de Cuiabá, process n° 443.830, November 2013, and had partnerships with the Instituto Lauro de Souza Lima, from São Paulo, Universidade de Cuiabá, and the Municipal Health Department of Cuiabá.
ResultsSeropositivity rate for anti-NDO-LID antibodies in children under 15 years of age was 4.0% among household contacts, 6.9% among neighborhood contacts, and 6.2% among all contacts together (household plus neighborhood contacts). Regarding household contacts, 62.0% lived with multibacillary leprosy patients (MBs) under 15 years of age, and 56.5% lived in neighborhoods within a radius of 100 meters from a bacilliferous child’s house. The description of the main sociodemographic and cohousing variables is shown in table 1.
Distribution of household and neighborhood contacts under 15 years of age according to sociodemographic, cohabitational, and laboratorial variables, Cuiabá-MT, 2016
| Variables | Household contacts (n = 50) | Neighborhood contacts (n = 160) | Total contacts (n = 210) | |||
|---|---|---|---|---|---|---|
| n | % | n | n | % | ||
| Age group | ||||||
| 1 – 7 years | 17 | 34.0 | 81 | 50.6 | 98 | 46.7 |
| 8 – 14 years | 33 | 66.0 | 79 | 49.4 | 112 | 53.3 |
| Sex | ||||||
| Male | 20 | 40.0 | 86 | 53.8 | 106 | 50.5 |
| Female | 30 | 60.0 | 74 | 46.2 | 104 | 49.5 |
| Race/Skin color | ||||||
| Brown | 29 | 58.0 | 96 | 60.0 | 125 | 59.5 |
| Non browna | 20 | 40.0 | 62 | 38.8 | 82 | 39.1 |
| Ignored | 1 | 2.0 | 2 | 1.2 | 3 | 1.4 |
| Residence area | ||||||
| Urban | 34 | 68.0 | 141 | 88.1 | 175 | 83.3 |
| Rural | 16 | 32.0 | 19 | 11.9 | 35 | 16.7 |
| # of people per household | ||||||
| 1 – 4 | 15 | 30.0 | 69 | 43.1 | 84 | 40.0 |
| ≥ 5 | 35 | 70.0 | 91 | 56.9 | 126 | 60.0 |
| # of people under 15 years in the house | ||||||
| ≥ 2 | 23 | 46.0 | 105 | 65.6 | 128 | 60.9 |
| > 2 | 27 | 54.0 | 55 | 34.4 | 82 | 39.1 |
| Positive serology | ||||||
| Yes | 2 | 4.0 | 11 | 6.9 | 13 | 6.2 |
| No | 48 | 96.0 | 149 | 93.1 | 197 | 93.8 |
In the bivariate analysis, no associations were found between the explanatory variables and seropositivity rate for anti-NDO-LID antibodies in relation to household contacts. Regarding neighborhood contacts, brown race/skin color was inversely associated with M. leprae infection when compared to the other races (PR = 0.24; 95% CI: 0.06-0.87). More than 5 people per household (PR = 7.85; 95% CI: 1.01-57.83) and households that had 2 or more children younger than 15 years (PR = 3.34; CI 95%: 1.02-10.92) were associated with the highest seropositivity rates for anti-NDO-LID antibodies (Table 2). The variables associated with the prevalence of infection by M. leprae in all contacts were brown race/skin color (PR = 0.26; 95% CI: 0.09-0.66), urban residence area (PR = 1.09; 95% CI: 1.03-1.14), 2 or more people under the age of 15 in the house (PR = 3.37; 95% CI: 1.05-8.56), and 5 or more people per household (PR = 7.58; 95% CI: 1.01-58.55).
Bivariate analysis of socioeconomic, demographic, and cohabitational variables associated with anti-NID-LID seropositivity in children under 15 years of age, contacts of leprosy patients, Cuiabá-MT, 2016
| Household contacts | Neighborhood contacts | All contacts | ||||
|---|---|---|---|---|---|---|
| Variables | PR-gross | IC 95% | PR-gross | IC 95% | PR-gross | IC 95% |
| Socioeconomic and demographic | ||||||
| Age (years) | ||||||
| 1 – 7 | - | 1.00 | 1.00 | |||
| 8 – 14 | - | 1.23 | 0.31 -3.86 | 1.40 | 0.47 -4.13 | |
| Sex | ||||||
| Male | 1.00 | 1.00 | 1.00 | |||
| Female | 0.66 | 0.04 -10.05 | 3.09 | 0.85 -11.25 | 3.18 | 0.87 -11.54 |
| Race/Skin colora | ||||||
| Non browna | - | 1.00 | 1.00 | |||
| Brown | - | 0.24 | 0.06 -0.87 | 0.26 | 0.09 -0.66 | |
| Residence area | ||||||
| Rural | - | 1.00 | 1.00 | |||
| Urban | - | 1.03 | 0.89 -1.62 | 1.09 | 1.03 -1.14 | |
| Housing situation | ||||||
| Own a home | - | 1.00 | 1.00 | |||
| Rent a house/ live for free | - | 0.35 | 0.04 -2.69 | 0.36 | 0.05 -2.69 | |
| Cohousing | ||||||
| # of people per household | ||||||
| 1 – 4 years | 1.00 | 1.00 | 1.00 | |||
| ≥ 5 | 0.42 | 0.02 -6.40 | 7.85 | 1.01 -57.83 | 7.58 | 1.01 -58.55 |
| # of people under 15 years in the house | ||||||
| ≥ 2 | 1.00 | 1.00 | 1.00 | |||
| > 2 | 0.85 | 0.05 -12.87 | 3.34 | 1.02 -10.92 | 3.37 | 1.05 -8.56 |
| Length of residencea | ||||||
| < 6 years | 1.00 | 1.00 | 1.00 | |||
| ≥ 6 years | 0.72 | 0.04- 10-93 | 0.51 | 0.14 -1.87 | 0.52 | 0.14 -1.87 |
The results of the final Poisson regression model can be visualized in table 3. The highest number of people per residence (PR = 8.71; 95% CI: 1.01-66.15) and brown race/skin color (PR = 0.21; 95% CI: 0.59-0.80) were associated with seropositivity rate for anti-NDO-LID antibodies in neighborhood contacts. In relation to the multiple model that evaluated all contacts together (household and neighborhood contacts), the variables associated with the prevalence of leprosy bacillus infection were female sex (PR = 1.05; 95% CI: 1.01-1.08), non-brown race/skin color (PR = 0.95; IC95%: 0.90-0.99), living in urban areas (RP = 1.05; 95% CI: 1.01-1.09), and a greater number of people per household (PR = 1.06; 95% CI: 1.02-1.08).
Final multiple regression model of the variables associated with seropositivity rate for anti-NID-LID antibodies in children under 15 years of age, contacts of leprosy patients, Cuiabá-MT, 2016
| Household contacts | Neighborhood contacts | All contacts | ||||
|---|---|---|---|---|---|---|
| Variables | PRadjust (CI 95%) | p-value | PRadjust (CI 95%) | p-value | PRadjust (CI 95%) | p-value |
| Sex | ||||||
| Male | - | - | 1.00 | |||
| Female | - | ns | - | ns | 1.05 (1.01 - 1.08) | 0.050 |
| Race/Skin colora | ||||||
| Non browna | - | 1.00 | 1.00 | |||
| Brown | - | ns | 0.21 (0.59 - 0.80) | 0.022 | 0.95 (0.90 - 0.99) | 0.027 |
| Residence area | ||||||
| Rural | - | - | 1.00 | |||
| Urban | - | ns | - | ns | 1.05 (1.01 - 1.09) | 0.005 |
| # of people per household | ||||||
| 1 - 4 | - | 1.00 | 1.00 | |||
| ≥5 | - | ns | 8.71 (1.01 - 66.15) | 0.049 | 1.06 (1.02 - 1.08) | 0.007 |
In this study, seropositivity rate for anti-NDO-LID antibodies in neighborhood contacts (6.9%) was close to that observed in other studies and may be justified by leprosy hyperendemicity in the region. A study carried out in an area endemic of Goiânia, state of Goiás, indicated a positive rate of 5.3%.9 In the hyperendemic area of Mato Grosso, the percentage of positive results to the rapid test was 14.7%.14 However, in these works, the positive results were observed in household contacts, and the majority of them were 15 years old or older.
The higher prevalence of leprosy infection observed in neighborhood contacts can be explained by the contact these children and adolescents have with infected patients in their neighborhood, school, or other nearby environments.4,16 A study to assess anti-PGL-I levels conducted in an endemic region of Rio de Janeiro revealed similar serological positivity rates between peridomiciliary contacts (15.6%) and household contacts (15.8%).17 In Minas Gerais, a higher serology response for anti-NDO-LID antibodies was observed in the general population when compared to household contacts.12 In this context, since positive test results may mean a higher risk of early manifestation of the disease – especially in multibacillary cases – epidemiological surveillance actions towards positive contacts may contribute to disrupt the transmission cycle and the development of the disease. Among these actions we can highlight bi-annual follow up for dermato-neurological evaluation, immunization with BCG vaccine, and screening of infected cases in the neighborhood and in schools.
The association between a greater number of people per household and urban residence with the highest seropositivity rates can be justified by the fact that precarious housing conditions and living in conglomerates of people are predictive factors for the development of leprosy, and express aspects of the social reality that circumscribe the disease occurrence.18-20 In fact, the literature indicates that the transmissibility and infectivity of M. leprae are higher in crowded places and may be related to physical and temporal proximity to the source of contamination.18,20,21 In this sense, the transmissibility of the disease was possibly influenced by the lower physical distances between untreated patients and susceptible individuals younger than 15 years of age. It may also have been influenced by precarious socio-sanitary conditions of the populations that live in the periphery of the cities. Moreover, it is pertinent to mention that individuals in the neighborhood played an important role in transmitting the infection to the child.3
We observed a prevalence of positive results among girls younger than 15 years of age. Similar to our research, a study carried out in the state of Minas Gerais, Brazil, with minors under 18 years of age, found a higher anti-PGL-I positivity among female contacts.22 Another study using PGL-I conducted among 6-18-year-old students and contacts of leprosy patients in an endemic region of the state of Pará found similar results in women.23 However, both studies failed to find statistically significant results. On the other hand, it should be mentioned that IgM levels tend to be higher in females than in males.24
The relationship observed in the bivariate analysis between seropositivity and the highest number of children under 15 years of age in the house, and the loss of association observed in the multiple analysis can be justified by the greater possibility of the family to have a greater agglomeration of people from other age groups. It is known that leprosy is common in people from disadvantaged social classes, with higher population densities per household, and insufficient education.16,23 The local epidemiological characteristics associated with the high magnitude of the disease in the region and the possibility of exogenous infection suggest regular exposure of the population to the bacillus. Thus, a high hidden prevalence of leprosy may be suspected.12,16 Moreover, the possibility of a greater susceptibility of children to leprosy infection due to immunological immaturity in this age group should also be considered.2,3
Self-reported brown skin color indicated a lower prevalence of leprosy when compared to non-brown skin color. A study carried out in the state of Mato Grosso in individuals with leprosy relapses found that the brown population was inversely associated with the higher prevalence of the disease. Therefore, the brown skin color seems to be a protection factor for the disease, in which relapses were 60% lower when compared to relapses in non-brown patients.15 Parsimony is recommended in the interpretation of these data since, apparently, there is no causal relation between race and disease resistance. Other factors, such as the higher prevalence of brown-skinned people in those states, may have influenced the outcome of the study. One possible interpretation that goes beyond the scope of the present study is that individuals with higher education levels tend to self-report their skin color more accurately.25 Another possible explanation lies in the fact that younger children may have been erroneously classified by parents or guardians in relation to skin color.
In the present study, the reduced sample size may have been a limitation for the identification of associated factors. It may also have contributed to the presence of wide confidence intervals. We also recommended parsimony in the interpretation of causality, since it is a cross-sectional study in which both exposure and outcome factors were determined simultaneously. However, this study is a pioneer in identifying factors associated with positive results for the anti-NDO-LID rapid test in contacts under 15 years of age. It may be useful in subsidizing health interventions for early diagnosis and control of the disease in this specific group.
ConclusionThis study identified high seropositivity rates for anti-NDO-LID antibodies in contacts of leprosy patients. Living with five or more people, female sex, non-brown skin color, and living in the urban area were variables associated with positive rapid anti-NDO-LID test results in children under 15 years of age.
Our results point to the need for continuous monitoring of positive leprosy contacts, with bi-annual dermato-neurological evaluations. We also recommend the implementation of a BCG vaccination program and tracking of infected populations and cases in the neighborhood and in schools as measures to control the disease.