Dear editor;
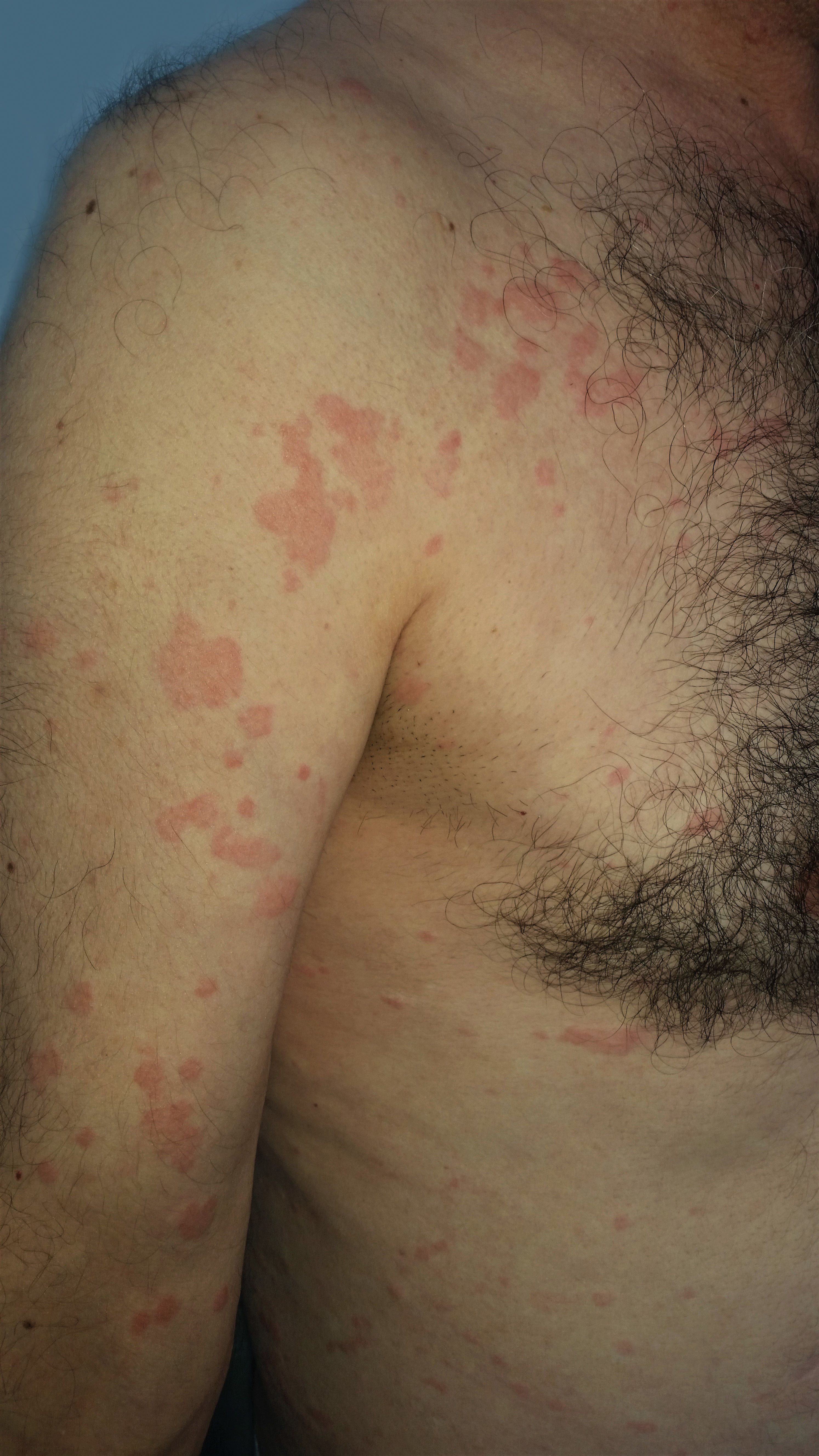
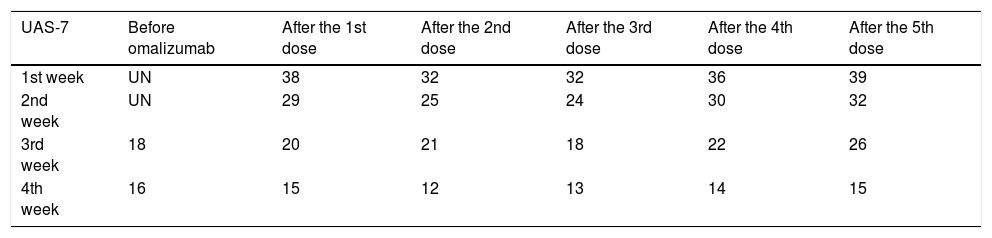
A 55-year-old man presented to our clinic with a 16-year history of chronic spontaneous urticaria (CSU). Given the poor response to H1-antihistamines at high doses alone or in combination with montelukast, he had started to receive omalizumab four months ago (300 mg/SC/every fourth week). He was referred to our clinic because of the exacerbation of his urticaria after this therapy. On physical examination, we detected the continuous eruption of short-lived hives on his body and extremities. He had no angioedema. His urticaria activity score-7 (UAS-7) was 14 in the preceding week. Complete blood count, liver, renal, and thyroid function tests, erythrocyte sedimentation rate, C3-C4 complement levels, and urine analysis were normal. Anti-thyroid and antinuclear antibodies, tests for human immunodeficiency, hepatitis B and C viruses, and stool examinations for parasitic infections were negative. Serum IgE level was 196 IU/mL (normal 0–87), and C-reactive protein (CRP) value was 2.2mg/L (normal<5 mg/L). His chest x-ray and abdominal ultrasonography were normal. Twenty-eight days after the last dose, we administered the fifth dose of omalizumab (300mg/SC) because of the ongoing symptoms. We also thought that the previous exacerbations should not be omalizumab-related. No acute reaction was observed following the administration, but an exaggeration of the urticaria and swelling of the lips and tongue were observed after about 12 hours (Figure 1). The CRP value measured in this period was 10.8mg/L. He was treated with antihistamines and systemic corticosteroids, but the UAS-7 was increased to 39 over the first week (Table 1). In the following period, the patient did not accept the diagnostic tests planned to determine the sensitizing compound of omalizumab.
The UAS-7 values of the patient several weeks before and after omalizumab
| UAS-7 | Before omalizumab | After the 1st dose | After the 2nd dose | After the 3rd dose | After the 4th dose | After the 5th dose |
|---|---|---|---|---|---|---|
| 1st week | UN | 38 | 32 | 32 | 36 | 39 |
| 2nd week | UN | 29 | 25 | 24 | 30 | 32 |
| 3rd week | 18 | 20 | 21 | 18 | 22 | 26 |
| 4th week | 16 | 15 | 12 | 13 | 14 | 15 |
Omalizumab is a recombinant DNA-derived humanized monoclonal antibody that selectively binds to human IgE. It was originally approved in 2003 for the treatment of persistent allergic asthma, and then was licensed in 2014 for the treatment of patients with antihistamine-refractory CSU. Over the past years, results of clinical studies and practical experience have shown its high efficiency with a rapid onset of action in CSU.1
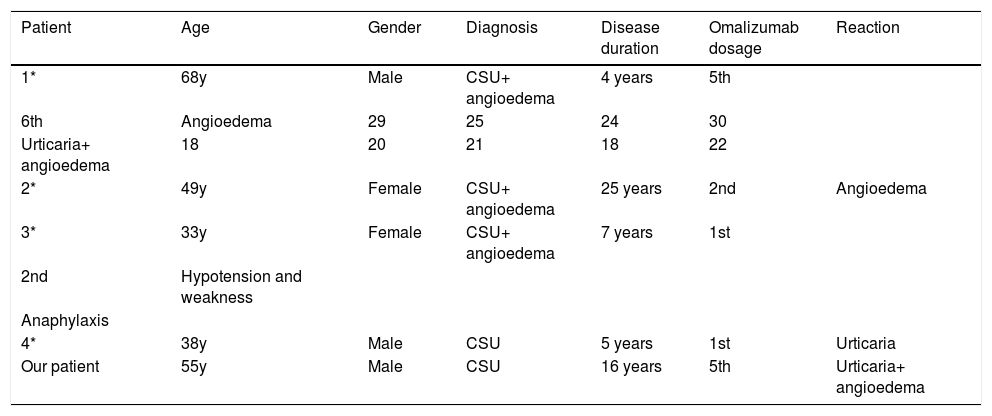
Omalizumab has been known to be associated with serious adverse reactions in patients with allergic asthma, but it has a better tolerability profile in patients with CSU. Most adverse reactions in CSU patients are mild and temporary such as fever, headache, sinusitis and reactions at the injection site.2 Although the failure of therapy has been rarely mentioned in some reports, exacerbation of urticaria associated with omalizumab is an unusual complication.3,4 Recently, Ertaş et al. reported four patients with severe antihistamine-resistant CSU, who developed angioedema, anaphylaxis and/or flare-up of urticaria at different times following omalizumab therapy (Table 2).4 In our patient, the exacerbation of urticaria and development of angioedema after the administration of the drug made us think that it was a paradoxical adverse reaction.
Exacerbation of urticaria and angioedema following omalizumab therapy
| Patient | Age | Gender | Diagnosis | Disease duration | Omalizumab dosage | Reaction |
|---|---|---|---|---|---|---|
| 1* | 68y | Male | CSU+ angioedema | 4 years | 5th | |
| 6th | Angioedema | 29 | 25 | 24 | 30 | |
| Urticaria+ angioedema | 18 | 20 | 21 | 18 | 22 | |
| 2* | 49y | Female | CSU+ angioedema | 25 years | 2nd | Angioedema |
| 3* | 33y | Female | CSU+ angioedema | 7 years | 1st | |
| 2nd | Hypotension and weakness | |||||
| Anaphylaxis | ||||||
| 4* | 38y | Male | CSU | 5 years | 1st | Urticaria |
| Our patient | 55y | Male | CSU | 16 years | 5th | Urticaria+ angioedema |
Even though we were not able to make the diagnostic tests, exacerbation of the pre-existing urticaria, and development of angioedema in our patient may be related with the excipients in omalizumab such as polysorbat and histidine rather than the active ingredient. Especially polysorbat is one of the well-established sensitizers that may be involved in the development of severe non-immunological reactions. Anaphylactoid reactions with cutaneous symptoms have also previously been described in asthmatic patients being treated with omalizumab.5 As most commercial preparations include different excipients which are necessary to preserve and stabilize the product, it should be taken into consideration that these excipients may play a role in the development of such adverse reactions and in unsatisfactory responses to the omalizumab therapy.
Financial support: None.
Conflict of interests: None.