More medication choices, extended treatment courses, and longer patient survival contribute to increased exposure to drugs and subsequently give rise to the incidence of Cutaneous adverse drug reactions (CADRs).1–3 Although most patients with CADRs will be cured after drug withdrawal, severe types of CADR require hospital interventions and are even life-threatening.4,5 This report retrospectively analyzed the characteristics of patients with CADRs based on hospitalized patients. Specifically, we focused on the Incubation period (IP) and associated factors.
A total of 308 confirmed patients with CADRs from 2013 to 2018 hospitalized at the First Affiliated Hospital of Chongqing Medical University were enrolled in this study. The demographic and clinical characteristics of these patients were collected from the electronic medical system. The relationship between IPs and other factors was analyzed by correlation analysis, and the differences in levels of IPs among different subgroups were compared by the Kruskal-Wallis test. This study was reviewed and approved by the Ethics Committee of the First Affiliated Hospital of Chongqing Medical University.
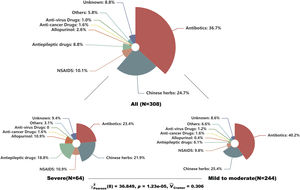
Table 1 showed the baseline characteristics of enrolled patients. The median age of enrolled patients was 47 yrs with 49.7% females. The results showed that erythema multiforme and maculopapular exanthema were the most common types, accounting for 30.5% and 26.6%, respectively. Severe CADRs like Steven-johnsons syndrome/Toxic epidermal necrolysis (SJS/TEN) and Drug reaction with eosinophilia and systemic symptoms (DRESS) covered 21% of them. The median IP was 4 days with a median length of stay of 7 days. Besides, skin lesions in about 40% of patients the mucosa was affected and over 70% of patients developed CADRs by means of oral administration. Regarding the culprit drugs, antibiotics were the most common drugs, covering 36.7% of all patients, followed by Chinses herbs (24.7%), non-steroidal anti-inflammatory drugs (10.1%), and anticonvulsants (8.8%). Furthermore, we compared the types of culprit drugs between patients with severe and mild-to-moderate CADRs. The result showed a significant difference between the two subgroups (p<0.001). Anticonvulsants and allopurinol seemed associated with severe types of CADRs (Fig. 1).
Clinical characteristics of patients with cutaneous adverse drug eruptions.
| All patients (n=308) | |
|---|---|
| Age, years | 47.0 (31.0‒62.0) |
| Gender, female | 153 (49.7%) |
| Diagnosis | |
| SJS/TEN | 47 (15.3%) |
| DRESS | 17 (5.5%) |
| Erythema multiforme | 94 (30.5%) |
| MPE | 82 (26.6%) |
| Fixed drug eruption | 54 (17.5%) |
| Others | 14 (4.5%) |
| Incubation period, days | 4.0 (1.0‒10.0) |
| IP of DRESS | 20.0 (4.0‒28.5) |
| Mucosa involvement | 128 (41.6%) |
| Length of stay, days | 7.0 (5.0‒11.0) |
| Comorbidities | |
| Hypertension | 50 (16.2%) |
| Diabetes | 46 (14.9%) |
| Chronic renal disease | 14 (4.5%) |
| Cancer | 10 (3.2%) |
| COPD | 7 (2.3%) |
| Autoimmune disease | 20 (6.5%) |
| Epilepsy | 16 (5.2%) |
| Culprit drug | |
| Antibiotics | 113 (36.7%) |
| Chinese herbsb | 76 (24.7%) |
| NSAIDSc | 31 (10.1%) |
| Anticonvulsantsd | 27 (8.8%) |
| Allopurinol | 8 (2.6%) |
| Anticancer drugs | 5 (1.6%) |
| Anti-virus drugs | 3 (1.0%) |
| Others | 24 (2.9%) |
| Unknown drugs | 21 (6.8%) |
| Route of administration | |
| Oral | 223 (72.4%) |
| Intramuscular injection | 2 (0.6%) |
| Intravenous injection | 62 (20.1%) |
| Unknown | 21 (6.8%) |
| Treatment | |
| Antihistamines alone | 11 (3.6%) |
| Cyclosporine alone | 2 (0.6%) |
| GC alone | 266 (86.4%) |
| GC+Azathioprine | 2 (0.6%) |
| GC+Cyclosporine | 9 (2.9%) |
| GC+IVIG | 3 (1.0%) |
| Glycyrrhizin alone | 9 (2.9%) |
| Methotrexate | 1 (0.3%) |
| Only supportive care | 5 (1.6%) |
COPD, Chronic Obstructive Pulmonary Disease; DRESS, Drug Reaction with Eosinophilia and Systemic Symptoms; GC, Glucocorticoids; IVIG, Intravenous Immunoglobulin; MPE, Maculopapular Exanthema; NSAIDS, Non-Steroidal Anti-Inflammatory Drugs; SJS, Stevens-Johnson Syndrome; TEN, Toxic Epidermal Necrolysis.
aAntibiotics include penicillins, macrolides, quinolones, cephalosporins, sulfonamides, aminoglycosides and miscellaneous antibiotics.
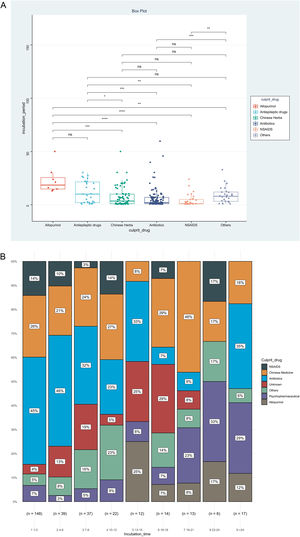
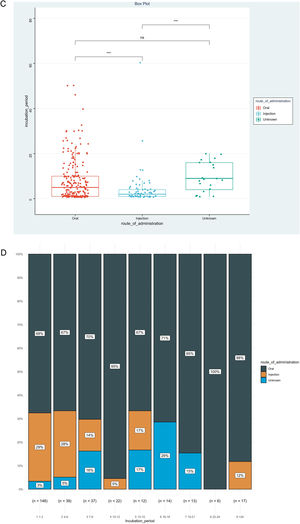
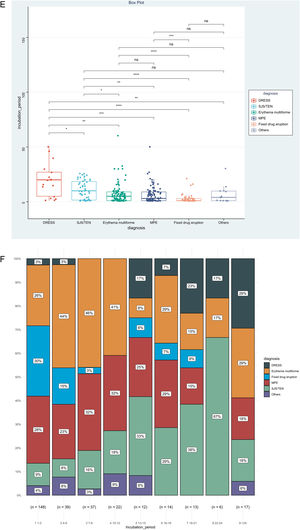
IP was the priority of this report, and we further analyzed factors associated with IP, as shown in Table 2. The result showed that disease types (χ2=0.153, p<0.001), route of administration (χ2=0.060, p<0.001), and culprit drugs (χ2=0.151, p<0.001) were the significantly correlated factors. However, no significant association could be detected between IPs and gender (rrank-biserial = 0.002, p=0.973), age (rho=0.104, p=0.068), and mucosa involvement (rrank-biserial = 0.132, p=0.054). Based on the results above, we then compared levels of IP among different subgroups (Fig. 2). Patients administered allopurinol and anticonvulsants had longer IPs than other drugs, oral administration longer than the injection, and severe CADRs longer than mild-to-moderate CADRs (p<0.05 for all, Fig. 2A‒C). The same association could also be seen if we presented the proportion of each subgroup by IP every 3 days (Fig. 2D‒F). The figure showed that although almost every subgroup could be detected in each group categorized by IP, the distribution of each subgroup was skewed and accumulated in certain IP categories, indicating the association between IP and these factors.
Levels and distributions of incubation period among subgroups of patients with cutaneous adverse drug reactions. The levels of the incubation period were compared using the Kruskal-Wallis test and the post hoc analysis was adjusted using the Bonferroni test. DRESS, Drug reaction with eosinophilia and systemic symptoms; MPE, Maculopapular exanthema; NSAIDS, Non-steroidal anti-inflammatory drugs; SJS, Stevens-johnson syndrome; TEN, Toxic epidermal necrolysis.
This study thoroughly analyzed the IPs of hospitalization-based CADRs and associated factors. However, the main limitation of this study is how to precisely determine the culprit drugs and IPs. Although IP and the culprit drug of each CADR patient were recorded in the electronic medical system, the nature of the retrospective design implied that the criteria were not unified. Consequently, the results may be biased.
In conclusion, this descriptive analysis suggested that severe and mild-to-moderate types of CADRs might be different diseases, especially in culprit drugs and IPs. Longer IPs were significantly associated with severe types, oral administration and allopurinol/anticonvulsants. This result may be helpful in understanding the IPs of CADRs and assessing the severity of CADRs.
Financial supportThis study was supported by the Postdoctoral Research Foundation of Chongqing Medical University (nº 2-01-02-04-P0474) and the Special Foundation for Postdoctoral Research Projects of Chongqing, Grant Number: 2021XM3080.
Authors’ contributionsXiaoli Chen: Methodology; data curation; visualization.
Li Hu: Conceptualization; project administration.
Zupeng Xiao: Resources; writing - review & editing.
Hanyi Wu: Validation; data curation.
Aijun Chen: Investigation; supervision.
Rentao Yu: Formal analysis; funding acquisition; software; roles/writing - original draft.
Conflicts of interestNone declared.
The patients in this manuscript have given written informed consent to the publication of their case details.
Study conducted at the First Affiliated Hospital of Chongqing Medical University, Chongqing, China.