Dear Editor,
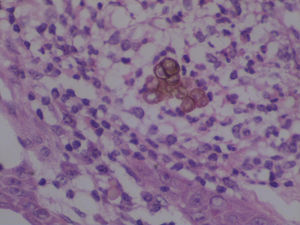
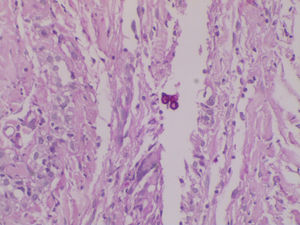
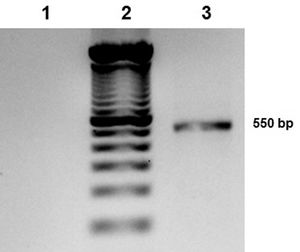
Chromoblastomycosis is a chronic, progressive, subcutaneous mycosis caused by dematiaceous (black) fungi clinically characterized by warty nodular lesions, generally on lower limbs but any region can be involved. The etiological agents mainly belong to three genera: Fonsecaea, Phialophora and Cladophialophora (Cladosporium). All of these pathogens are characterized by parasitic structures known as fumagoid cells or Medlar bodies. Most of affected individuals are rural workers who have a history of local trauma with decaying plant debri and frequently wear inadequate footwear, such as sandals. In 2010, we published a clinical case regarding a patient with squamous cell carcinoma secondary to longstanding lesions of chromoblastomycosis, who died due to multiorgan failure with hydroelectrolytic imbalance.1 Based on phenotypic fungal characteristics, the culture had been previously identified as Fonseaea pedrosoi. The culture was stored and now, ten years after the case release, we carried out polymerase chain reaction (PCR) amplification and sequencing of internal transcribed spacer (ITS) and ribosomal DNA (rDNA; Figures 1 and 2).2 The 550 bp PCR product was purified, sequenced in both directions and submitted to the BLAST database at NCBI. The sequence showed a 100% homology with Cladosporium langeronii DTO-124-D5, R1, CBS189.54, among others (Figure 3).
In 2006, Zalar et al. described C. langeronii, along with seven new species sampled from a variety of substrates, as species related to the ubiquitous Cladosporium sphaerospermum. This description and phylogenetic analysis was based on the amplification and sequence of the rDNA-ITS region.3Cladosporium species are particularly distinguished by their strikingly slow-growing colonies in potato dextrose agar, malt extract agar and oat agar at 25°C in the dark with a maximum salt concentration of 17%. Cladosporium develop dark-brown smooth oblong conidia with light ornamentations (minutely verruculose spores) and scarce secondary ramoconidia.4Cladosporium are ubiquitous without any apparent predilection for a specific environment. Its habitat comprises polar ice, decomposing plant matter, window frames, nasal mucus, and more recently, water from the Caspian Sea.5 Clinical cases are suspected to be accidental as a consequence of traumatism involving contaminated materials.
The classical identification based on macroscopic and microscopic characteristics provides a weak diagnosis. Currently, molecular techniques based on the rDNA-ITS region of Cladosporium allow more precise species identification. Thus, we communicate the molecular confirmation C. langeronii as the causative agent of the 2007 clinical case. So far, only three sequences of C. langeronii rDNA-ITS have been reported associated with human cases. The first sequence, CBS189.54 NT (access number DQ780379), is associated with mycosis in Brazil, while the other two sequences (access numbers AF4555254 and AY3453524) are related to nasal mucosa.3 We believe communicating the molecular confirmation of a human disease caused by this extremely rare agent is important.
Financial support: None.
Conflict of interests: None.